
Wojciech Wojakowski, MD
Medical University of Silesia in Katowice, Poland
Renowned cardiologist, Wojciech Wojakowski, MD (Medical University of Silesia in Katowice, Poland) sheds light on technical considerations and execution of the transcatheter paravalvular leak (TPVL) procedure during a recent medical conference.
Paravalvular leaks (PVLs) are serious complications that can occur after surgical valve replacement or transcatheter aortic valve replacement (AVR). TPVL closure offers a less invasive alternative to surgical re-intervention. The safety and feasibility of TPVL closure have been validated through several registries and a meta-analysis.
Wojakowski shared valuable insights regarding technical considerations and recommendations for the TPVL procedure on May 8th, 2023, at the TCTAP conference.
"PVL occurs due to various factors such as the type of prosthesis and endocarditis," explained Wojakowski. "The overall incidence of PVLs ranges from 5% to 18%, with 74% occurring within the first-year post-operation and 16% appearing later due to suture dehiscence associated with subacute bacterial endocarditis."
Indications for TPVL Closure
Wojakowski emphasized that TPVL is contraindicated in the presence of valve rocking/instability or active infective endocarditis. However, the procedure can be performed when heart failure symptoms (NYHA II-IV class) and/or hemolysis are present, along with a PVL jet of Grade 2 or higher observed in color Doppler (color coded Doppler flow mapping) on echocardiography. Additionally, specific parameters related to the valves need to be identified. For mitral PVL, these parameters include systolic flow reversal in the pulmonary vein, increased pulmonary artery pressure, lack of left atrium (LA) size reduction, or progressive LA dilatation after mitral valve replacement (MVR), and high forward transprosthetic flow velocity. In the case of aortic PVL, parameters, such as holodiastolic flow reversal in the descending aorta, lack of left ventricle (LV) size reduction, or progressive LV dilatation after AVR, and high forward transprosthetic flow velocity, should be assessed.
Diagnosis of PVL
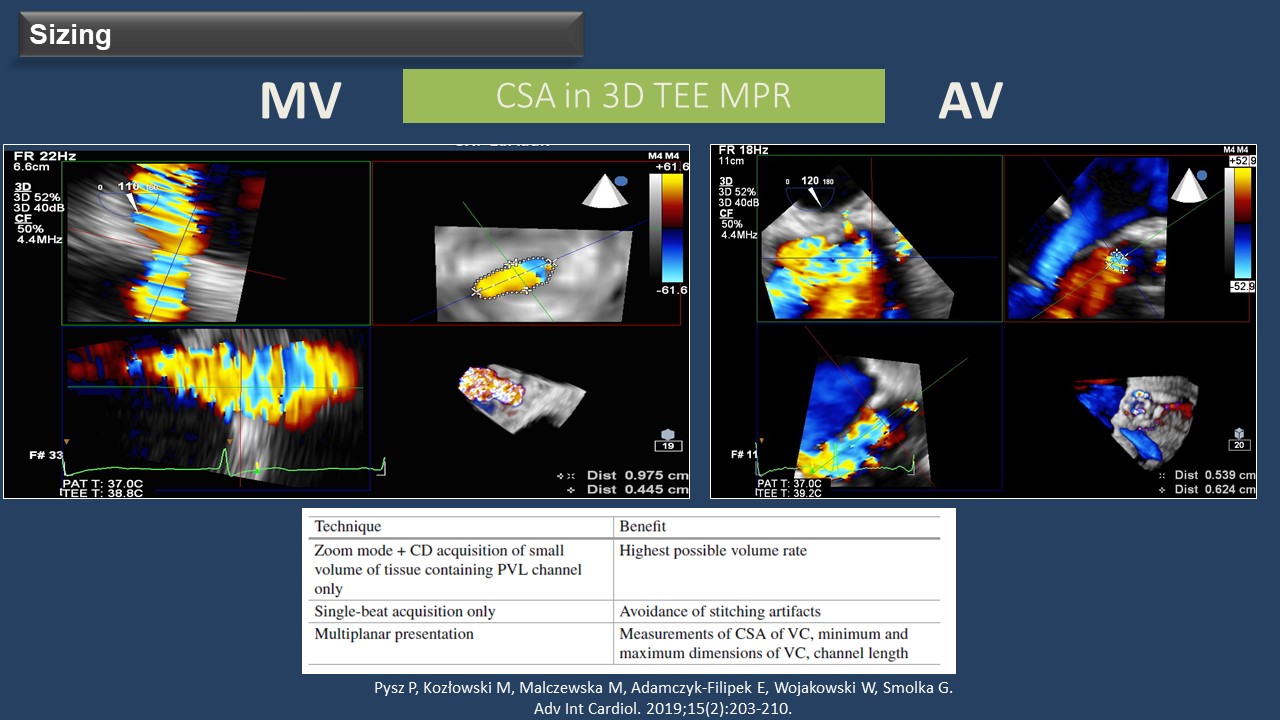
When PVL is suspected, an echocardiography study is crucial to confirm the diagnosis. Three-dimensional transesophageal echocardiography (3D-TEE) allows for a better definition of the PVLs, assessing their number, shape, and location, making it the gold standard for PVL evaluation. "For PVL sizing, the cross-sectional area can be measured in 3D-TEE multiplanar reconstruction, using single-beat acquisition only," added Wojakowski. TEE plays a crucial role during the TPVL closure procedure, and 3D-TEE is essential for device selection, procedure guidance, and result assessment. Additionally, fusion imaging techniques automatically fusing live 3D-TEE and live X-rays in real-time can provide valuable assistance during procedures such as a transseptal puncture or crossing the PVL with the guide wire.
When it comes to aortic PVL closure, the retrograde approach is commonly used for most cases, while the anterograde approach with an apical puncture is rarely employed. Wojakowski explained the "mother-and-child technique" which involves using a diagnostic catheter within a guiding sheath. After crossing the defect, a hydrophilic guidewire is replaced with a stiffer wire, and the device is finally deployed after advancing the delivery sheath.
In the case of mitral PVL closure, the anterograde approach with transseptal puncture is mostly utilized, while the retrograde approach with transaortic or transapical puncture is seldom used, particularly for posterior or medial mitral PVL. Wojakowski emphasized that the location of the septal puncture depends on the PVL's location and should be guided by TEE. In certain instances, such as septal PVL or unfavorable angles between the transseptal puncture and the defect, using a deflectable catheter like the Occlutech steerable guiding sheath can be highly beneficial.
Current Device Selection
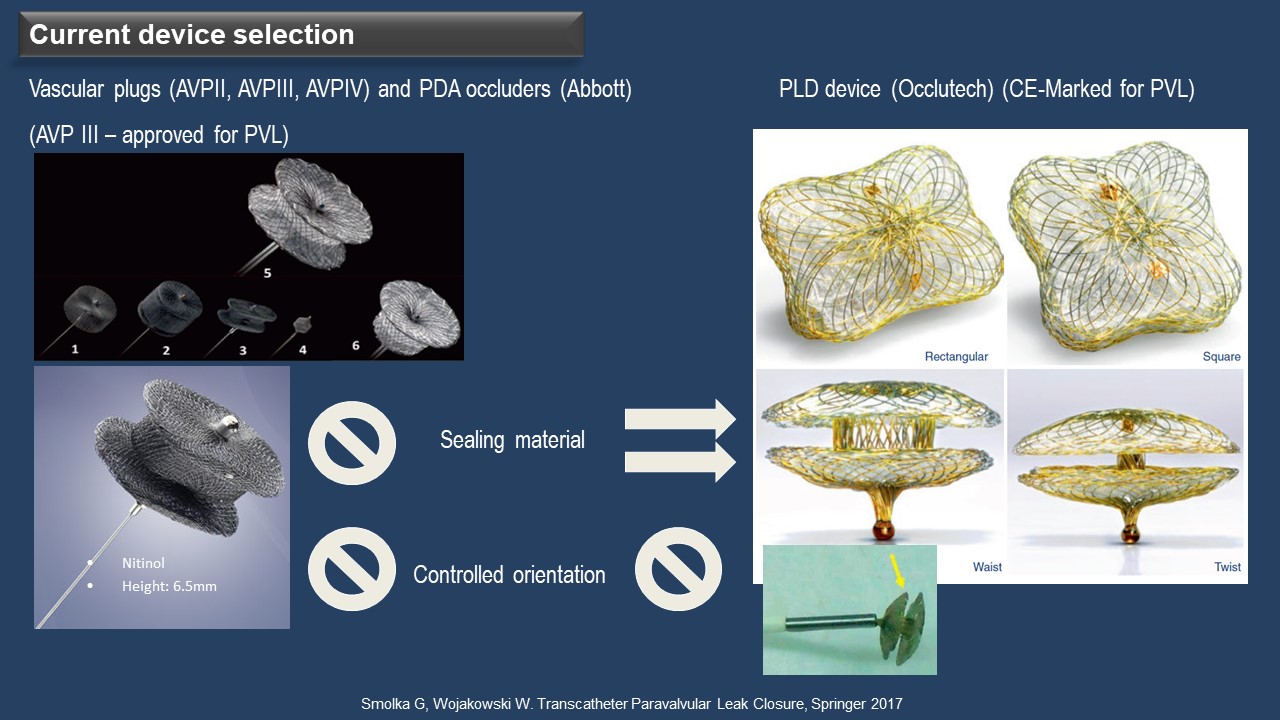
PVLs vary in size and shape, often appearing crescentic and serpiginous rather than cylindrical, making it challenging to find a single device suitable for all cases. The Amplatzer Vascular Plug (AVP) III is widely used and approved for PVL in Europe. The Occlutech Paravalvular Leakage Devices (PLD) have received a CE-marking for PVL. According to Wojakowski, AVP III lacks sealing materials on both sides of the disc, allowing for oversized use in irregular or crescentic PVL and multiple PVLs (Figure 2). On the other hand, PLD features sealing materials on both sides of the discs, not permitting oversizing and enabling use in round or oval PVL but limited to single PVL cases.
Post-Procedural Assessment and Follow-Up
Multiple registries and a meta-analysis have confirmed the safety and feasibility of TPVL closure. However, there is a risk of residual or recurrent leak and hemolysis, and incomplete closure, despite reducing heart failure symptoms, may lead to a worsening of hemolysis. Wojakowski highlighted factors influencing hemolysis recurrence after TPVL, including the amount of remaining paravalvular leakage (less than 90% of the cross-sectional area), mitral location, and calcification. Post-procedural imaging with TEE is recommended to assess device position and residual regurgitation, utilizing color Doppler imaging and specific parameters such as pulse wave Doppler of the pulmonary vein for mitral PVL.
Future Directions
Wojakowski concluded that transcatheter closure of PVL is the preferred treatment for patients with heart failure or hemolytic anemia compared to surgical re-intervention, offering lower procedural morbidity and mortality. However, to further enhance procedural success and outcomes, there is a need for dedicated devices that provide complete sealing..
Meet the Experts over Breakfast
Mitral & Tricuspid Valve Intervention
Monday, May 8, 7:00 AM - 8:00 AM
Valve & Endovascular Theater, Vista 1, B2
Edited by

Sang-Cheol Cho , MD
Yeosu Jeil Hospital, Korea (Republic of)

