
Seung-Jung Park, MD
Asan Medical Center, Korea (Republic of)
Acute coronary syndrome is commonly caused by the rupture of vulnerable plaque, which often has a mild angiographic appearance. Although systemic pharmacologic management is considered a standard therapy for stabilizing vulnerable plaque, the role of the local treatment to prevent plaque rupture has not yet been determined and has only been tested in small trials. The PREVENT trial is designed to compare the effectiveness of preventive percutaneous coronary intervention (PCI) plus optimal medical therapy (OMT) to OMT alone in patients with functionally insignificant, high-risk vulnerable plaques.
The PREVENT trial is a multinational, multicenter, prospective, open-label, active-treatment-controlled randomized clinical trial (Figure 1). Patients with at least one angiographically significant stenosis (diameter stenosis >50%) without functional significance (fractional flow reserve [FFR] >0.80) and vulnerable plaque characteristics in intracoronary imaging are eligible for enrollment. Target lesions should have at least two of four intracoronary imaging criteria for vulnerable plaque; (1) minimal lumen area (MLA) <4.0 mm2; (2) plaque burden >70%; (3) maximal lipid core burden index (LCBI) in a 4 mm segment >315 by near-infrared spectroscopy (NIRS); and (4) thin cap fibroatheroma (TCFA) as determined by optical coherent tomography (OCT) or virtual histology (VH). Enrolled patients were randomly assigned in a 1:1 ratio to either a preventive PCI on vulnerable plaque using bioabsorbable vascular scaffolds or metallic everolimus-eluting stents plus OMT or OMT alone. The primary endpoint is a target-vessel failure, defined as a composite of death from cardiac causes, target-vessel myocardial infarction, ischemic-driven target-vessel revascularization, and hospitalization for unstable or progressive angina at 2 years.
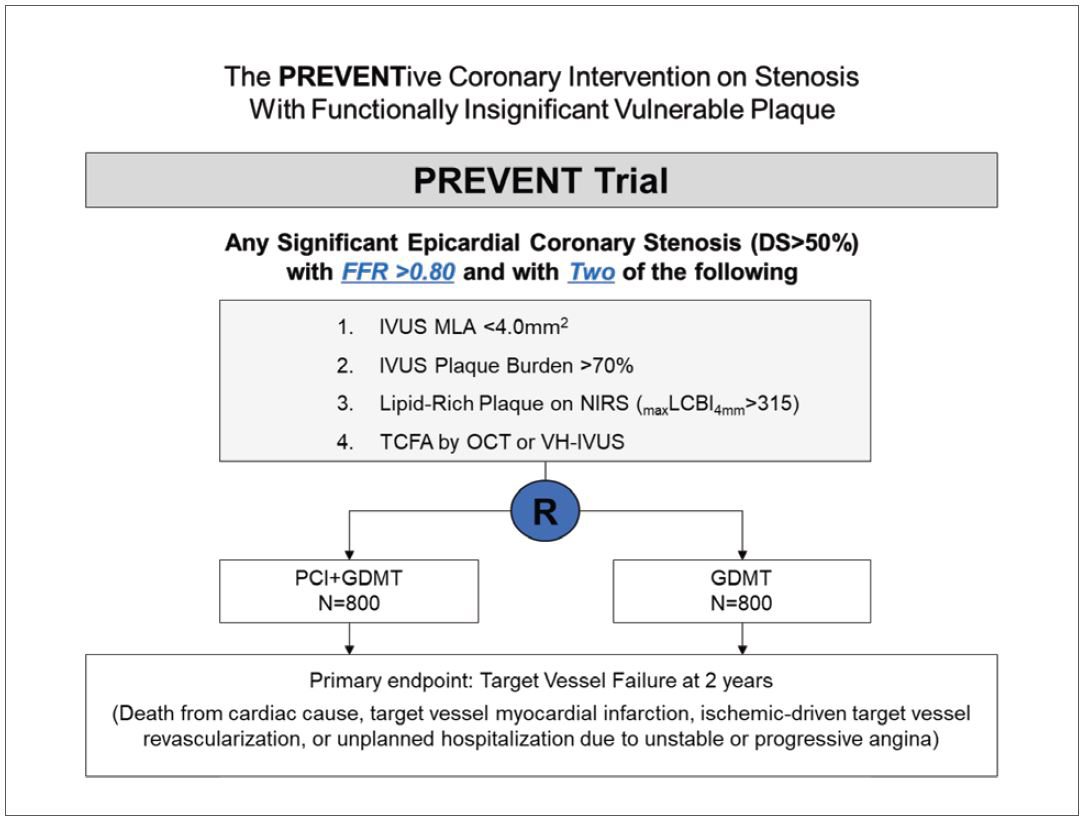
Enrollment of a total of 1,608 patients has been completed. Follow-up of the last enrolled patient will be completed in September 2023 and primary results will be available by early 2024. The PREVENT trial is the first, a large-scale randomized trial, with adequate power for clinical outcomes to evaluate the effect of preventive PCI on non-flow-limiting vulnerable plaque with high-risk features. The results of this trial will provide compelling evidence to determine whether preventive PCI of focal vulnerable plaques on top of OMT improves patient outcomes compared to OMT alone.
Clinical Science
Future Perspective on Ongoing Trials from AMC
Monday, May 08, 11:00 AM - 12:20 AM
Presentation Theater 1, Vista 3, B2
Edited by

Do-Yoon Kang, MD
Asan Medical Center, Korea (Republic of)

