Lots of interesting abstracts and cases were submitted for TCTAP 2023. Below are the accepted ones after a thorough review by our official reviewers. Don’t miss the opportunity to expand your knowledge and interact with authors as well as virtual participants by sharing your opinion in the comment section!
TCTAP C-172
Triglyceride Deposit Cardiomyovasculopathy, a Novel Heart Disease Refractory to Conventional Treatment in Patients With Cardiac Ischemia and Cardiac Dysfunction Diagnosed With BMIPP Scintigraphy
By Atomu Tajima, Yusuke Nakano, Akihiro Suzuki, Kento Kikuchi, Masanobu Fujimoto, Hirohiko Ando, Tetsuya Amano
Presenter
Atomu Tajima
Authors
Atomu Tajima1, Yusuke Nakano1, Akihiro Suzuki1, Kento Kikuchi1, Masanobu Fujimoto1, Hirohiko Ando1, Tetsuya Amano1
Affiliation
Aichi Medical University, Japan1,
View Study Report
TCTAP C-172
OTHERS - Others (Unclassified)
Triglyceride Deposit Cardiomyovasculopathy, a Novel Heart Disease Refractory to Conventional Treatment in Patients With Cardiac Ischemia and Cardiac Dysfunction Diagnosed With BMIPP Scintigraphy
Atomu Tajima1, Yusuke Nakano1, Akihiro Suzuki1, Kento Kikuchi1, Masanobu Fujimoto1, Hirohiko Ando1, Tetsuya Amano1
Aichi Medical University, Japan1,
Clinical Information
Patient initials or Identifier Number
S.M.
Relevant Clinical History and Physical Exam
A 72-y-o male visitedour hospital due to orthopnea. Heart failure (HF) was suspected and admittedfor close examination. He had a history of COPD, type 2 diabetes, dyslipidemia,and smoking 2 packs/day. The therapeutic agents were administered: insulin, beta-blocker,and loop diuretics. Physical examination: Height 168 cm; Body weight 68 kg;Blood pressure 142/85 mmHg; Pulse 100 bpm; BT 36.1 ℃; SpO2 90 % (room air); With a cardiac murmur,moist rales at respiration, and leg edema.
Relevant Test Results Prior to Catheterization
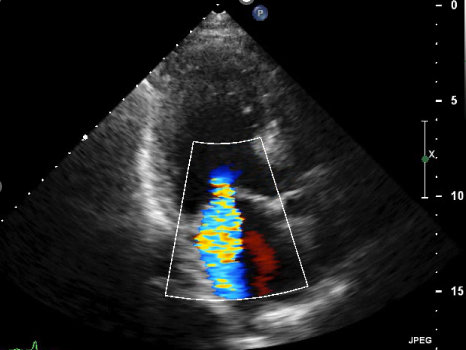
No apparentabnormality, except for BNP 1901 pg/mL, HbA1c 8.3 %, BUN 27 mg/dL, and creatinine1.03 mg/dL were detected by blood tests. Chest X-ray showed emphysematouschanges and bilateral pleural effusions in the lung fields without cardiomegaly.ECG showed sinus 110 bpm, and horizontal ST depression at chest leads.Echocardiography revealed LVFE 26.9 %, dilated left ventricle, moderate-severeMR, TRPG 33.0 mmHg. The patient's condition improved with standard HFtreatment.
Relevant Catheterization Findings
Coronary angiographyshowed severe significant stenosis in all coronary vessels (#4AV 90%, #6-7 75 %,#9 75 %, HL 90%, #13 90 %) with FFR 0.78 and RFR 0.81 in LAD. Swan-Gantzcatheter showed increased left ventricular end-diastolic pressure of PCWP 17 mmHg.
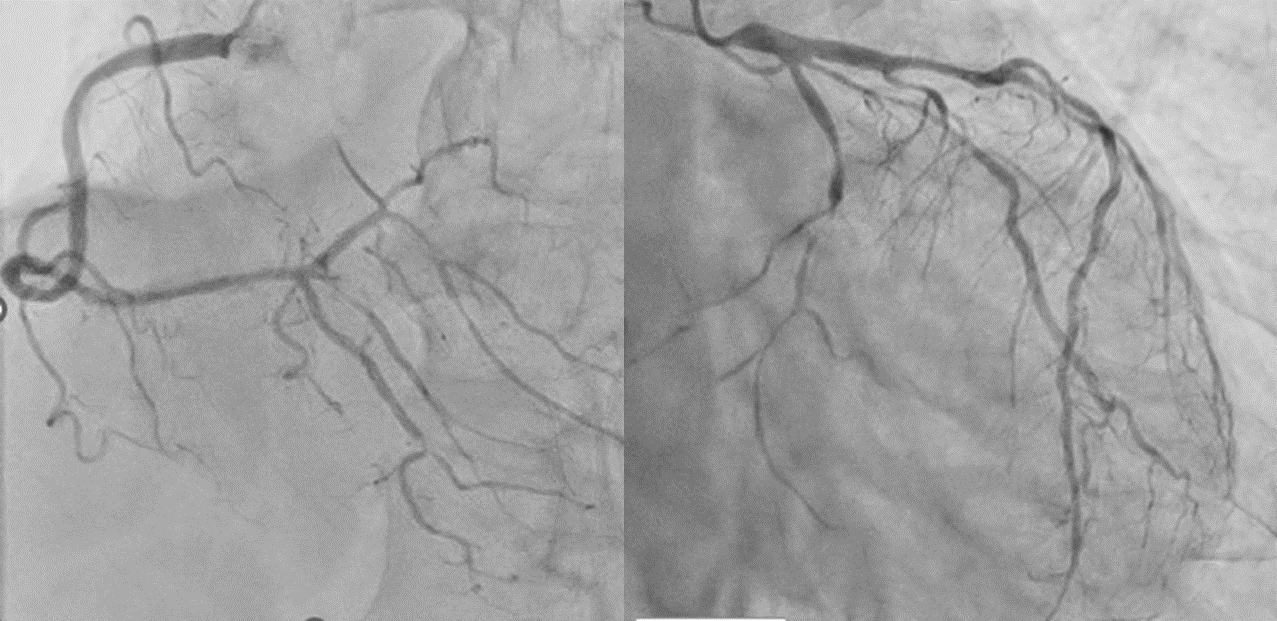

Interventional Management
Procedural Step
Echocardiography after treatment for HF revealedthat EF remained unchanged in the 20 % range and severe MR remained, although myocardialviability was confirmed by scintigraphy. Based on multivessel coronary stenosisfindings and decreased wash-out rate of less than 10 % in123I-β-methyl-P-iodophenyl-pentadecanoic acids (BMIPP) scintigraphy, whichsuggests abnormal fatty acid metabolism, a diagnosis of triglyceride depositcardiomyovasculopathy (TGCV) was made. TGCV is a cardiovascular disorder inwhich the defective hydrolysis of intracellular triglycerideleads to a novel form of diffuse, narrowing atherosclerosis causingtriglyceride deposition in the smooth muscle and endothelial cells, as againstatherosclerosis induced by hypercholesterolemia presenting with focal andeccentric stenosis.
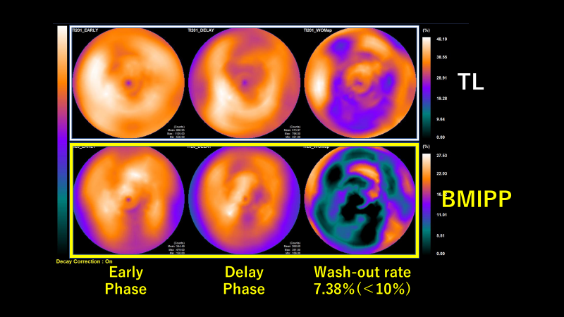


Case Summary
We experienced a case of TGCV with severecoronary artery stenosis and reduced cardiac function. The patient's conditiondid not improve even after invasive therapy, so TGCV needs to be recognized as anovel risk factor refractory to conventional treatment in patients with cardiacischemia and cardiac dysfunction. The effectiveness of the novel treatment drugcontaining tricaprin/trisdecanoin is currently being tested in phase IIa/IIIclinical trials.


