Lots of interesting abstracts and cases were submitted for TCTAP 2023. Below are the accepted ones after a thorough review by our official reviewers. Don’t miss the opportunity to expand your knowledge and interact with authors as well as virtual participants by sharing your opinion in the comment section!
TCTAP C-141
Consideration for Coronary Intervention in a Patient Ankylosing Spondylitis With Anti-TNF Monoclonal Antibody Therapy
By Antonia Anna Lukito, Sonia Chandra, Riyandi Ardi Putra Fernandes, Hardi Hutabarat, Audie Christopher
Presenter
Hardi Hutabarat
Authors
Antonia Anna Lukito1, Sonia Chandra1, Riyandi Ardi Putra Fernandes1, Hardi Hutabarat1, Audie Christopher1
Affiliation
Siloam Hospitals Lippo Village, Indonesia1,
View Study Report
TCTAP C-141
CORONARY - Stents (Bare-metal, Drug-eluting)
Consideration for Coronary Intervention in a Patient Ankylosing Spondylitis With Anti-TNF Monoclonal Antibody Therapy
Antonia Anna Lukito1, Sonia Chandra1, Riyandi Ardi Putra Fernandes1, Hardi Hutabarat1, Audie Christopher1
Siloam Hospitals Lippo Village, Indonesia1,
Clinical Information
Patient initials or Identifier Number
Mrs. FW
Relevant Clinical History and Physical Exam
A 52-year-old female presented with angina and back pain for 1 month. CVRFs are hypertension, diabetes, dyslipidemia, and ankylosing spondylitis (HLA-B27 positive) on Golimumab (anti TNF monoclonal antibody) therapy. History of total hysterectomy and oophorectomy bilateral since 5 years ago.
Relevant Test Results Prior to Catheterization
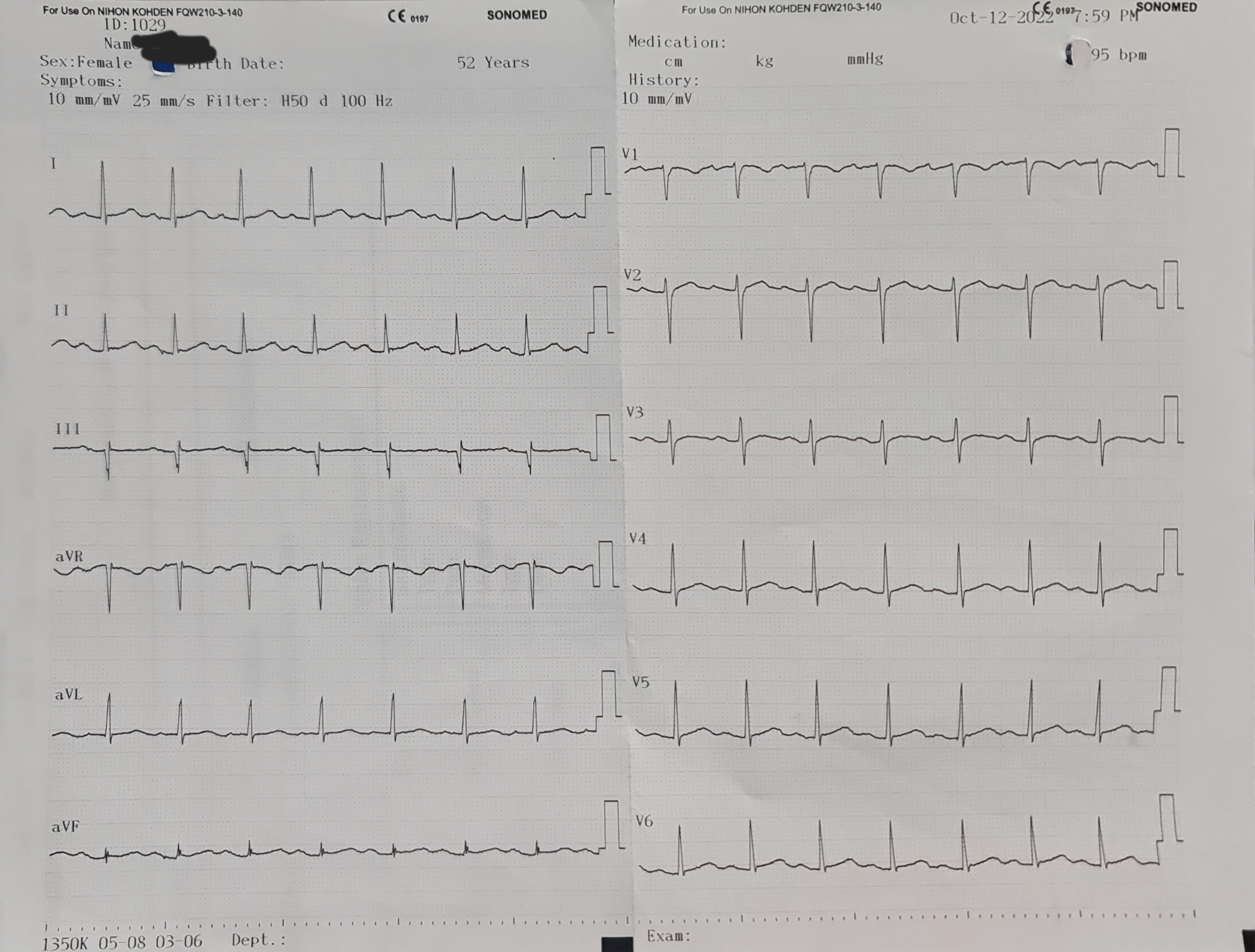
ECG showed normal sinus rhythm and no ST-T segment changes. The CCTA showed three vessels disease with calcium score of 470. Echocardiography revealed no RWMA, with LVEF of 70%.

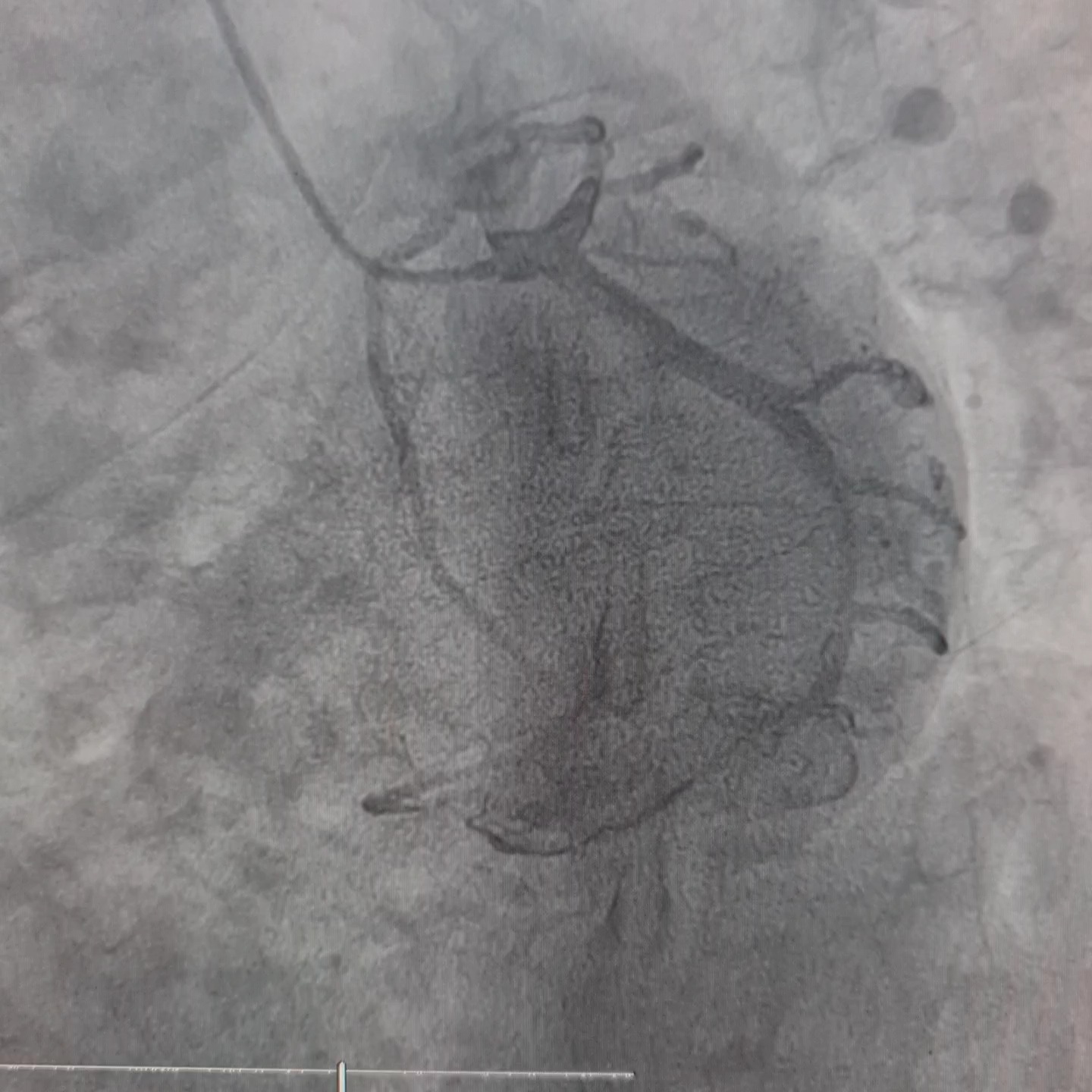
Relevant Catheterization Findings
Coronary angiography revealed left dominant system and three vessels disease. RCA was non dominant and small in caliber, with 40-50% lesion at mid-distal segment.
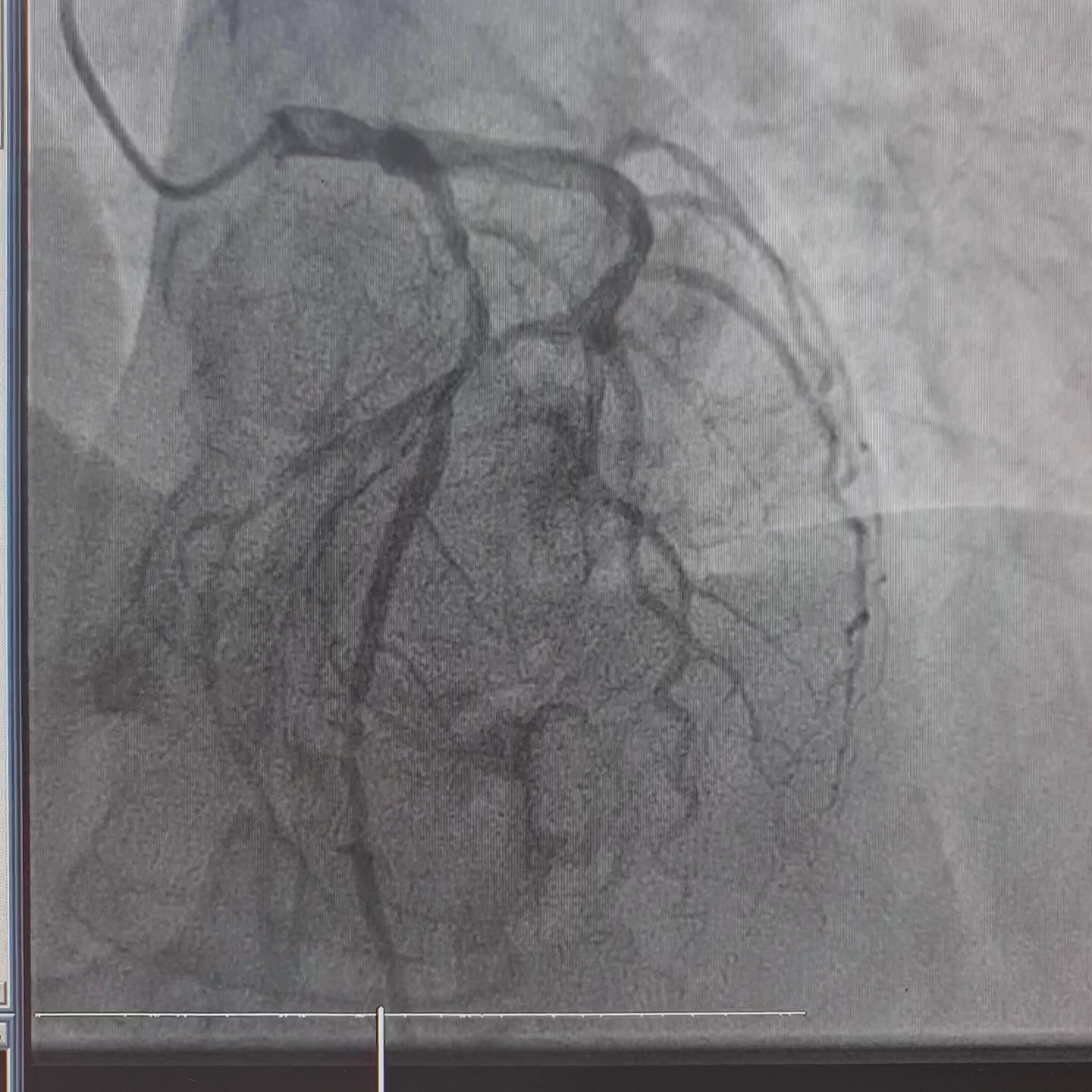
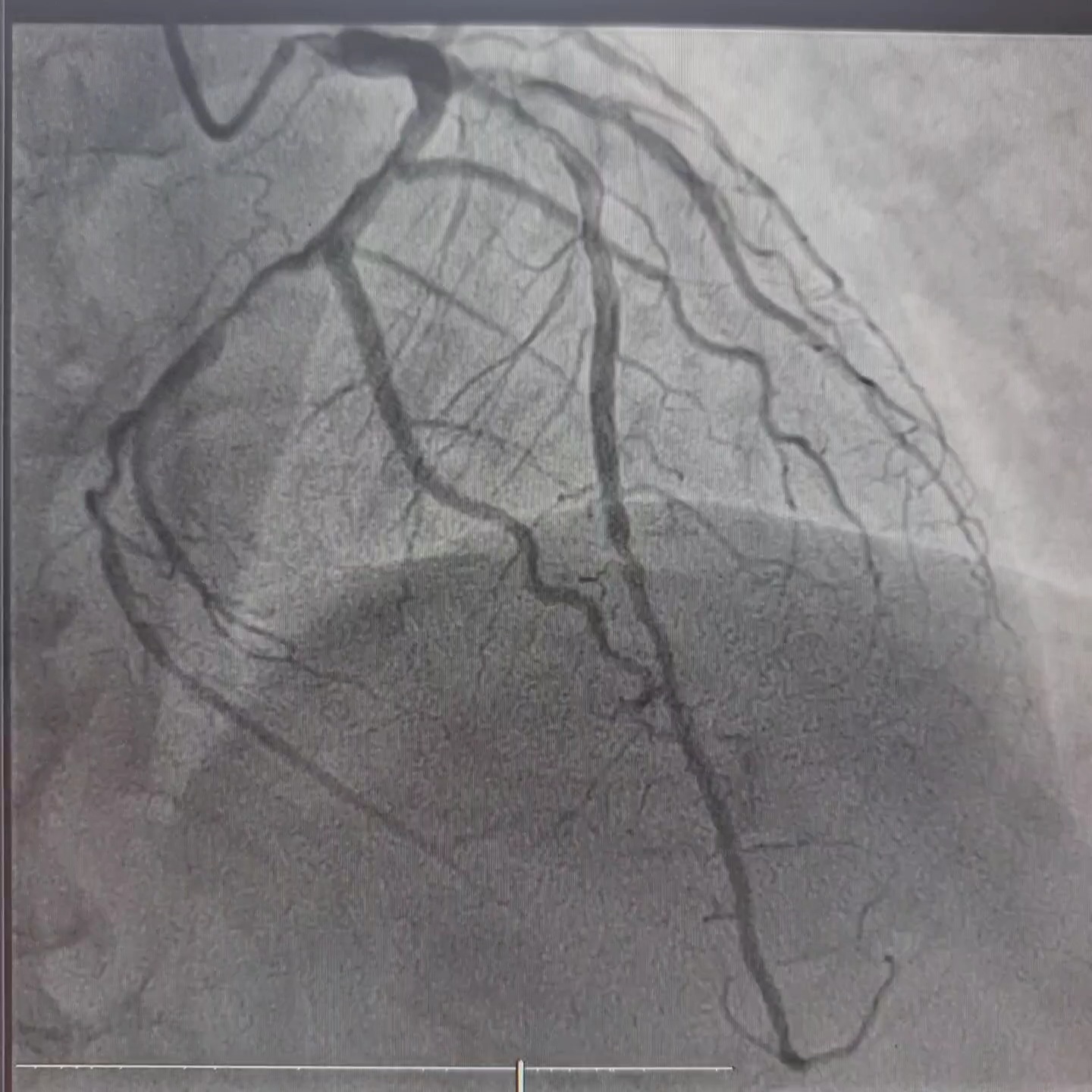



Interventional Management
Procedural Step
Patient was agreed for further intervention procedure.
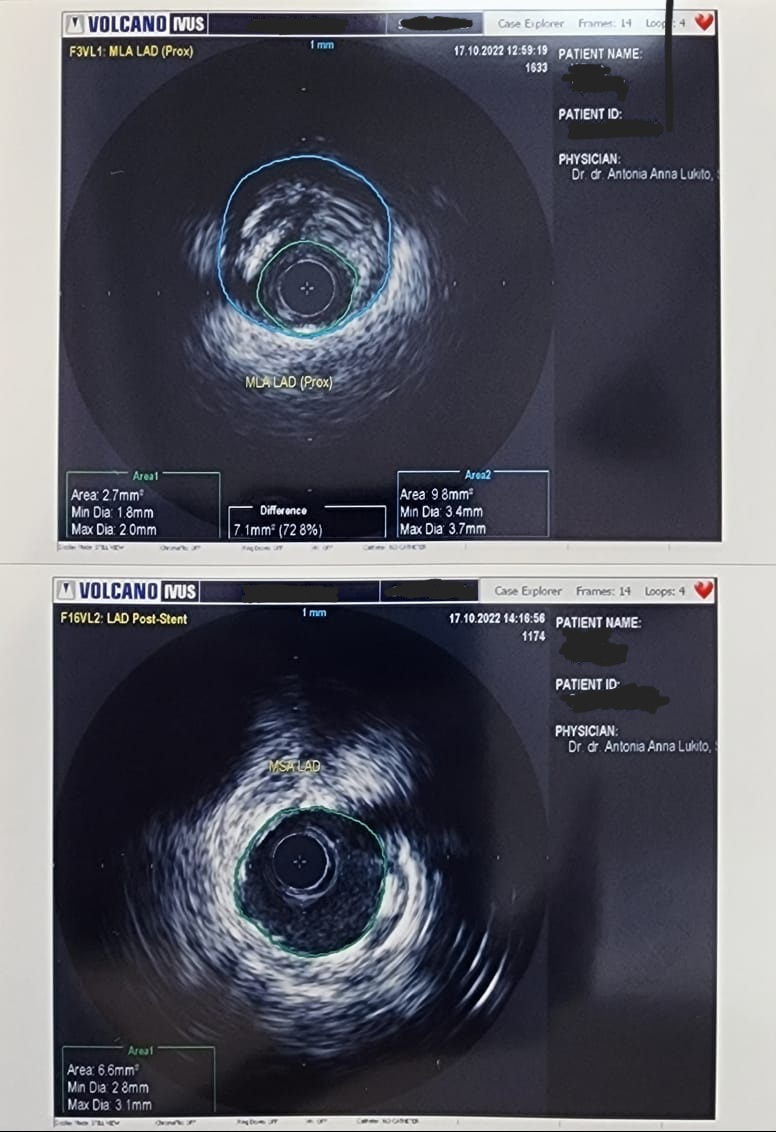
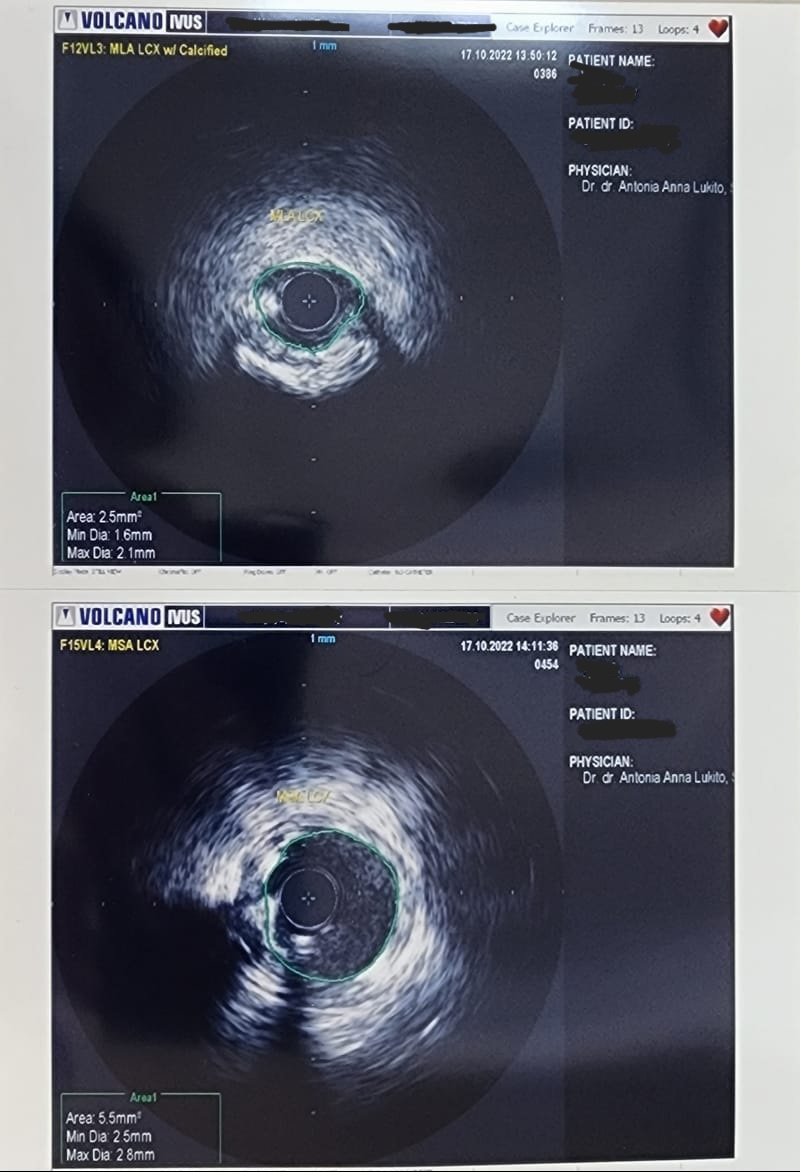



Case Summary
The consideration for choosing short-DAPT-approved DES for this patient that has the comorbidity of ankylosing spondylitis which need the long-term anti TNF monoclonal antibody therapy (Golimumab) that carries higher risk of bleeding. And second consideration for using IVUS guided DES implantation were for accurate stent sizing and to ensure well-deployed stent and no edge dissection to avoid the potential of both in-stent restenosis and in-stent thrombosis.


