Lots of interesting abstracts and cases were submitted for TCTAP 2022. Below are the accepted ones after a thorough review by our official reviewers. Don’t miss the opportunity to expand your knowledge and interact with authors as well as virtual participants by sharing your opinion in the comment section!
TCTAP A-089
PREmier of Randomized COMparison of Bypass Surgery Versus AgnioplasTy -5 Biodegradable Polymer Sirolimus-Eluting Stent in Patients With Left Main Coronary Artery Disease: PRE-COMBAT-5 Trial
By Jinho Lee, Do-Yoon Kang, Jung-Min Ahn, Duk-Woo Park, Seung-Jung Park
Presenter
Jinho Lee
Authors
Jinho Lee1, Do-Yoon Kang2, Jung-Min Ahn2, Duk-Woo Park2, Seung-Jung Park2
Affiliation
Kyung Hee University Medical Center, Korea (Republic of)1, Asan Medical Center, Korea (Republic of)2
View Study Report
TCTAP A-089
Stents (Bare-metal, Drug-eluting)
PREmier of Randomized COMparison of Bypass Surgery Versus AgnioplasTy -5 Biodegradable Polymer Sirolimus-Eluting Stent in Patients With Left Main Coronary Artery Disease: PRE-COMBAT-5 Trial
Jinho Lee1, Do-Yoon Kang2, Jung-Min Ahn2, Duk-Woo Park2, Seung-Jung Park2
Kyung Hee University Medical Center, Korea (Republic of)1, Asan Medical Center, Korea (Republic of)2
Background
Newer drug-eluting stent (DES) with biodegradable polymer has been introduced to reduce the potential stent thrombosis related to durable polymers. The safety and efficacy of the biodegradable polymer sirolimus-eluting Orsiro stent (SES) in the treatment of unprotected left main coronary artery (ULMCA) stenosis has not been determined.
Methods
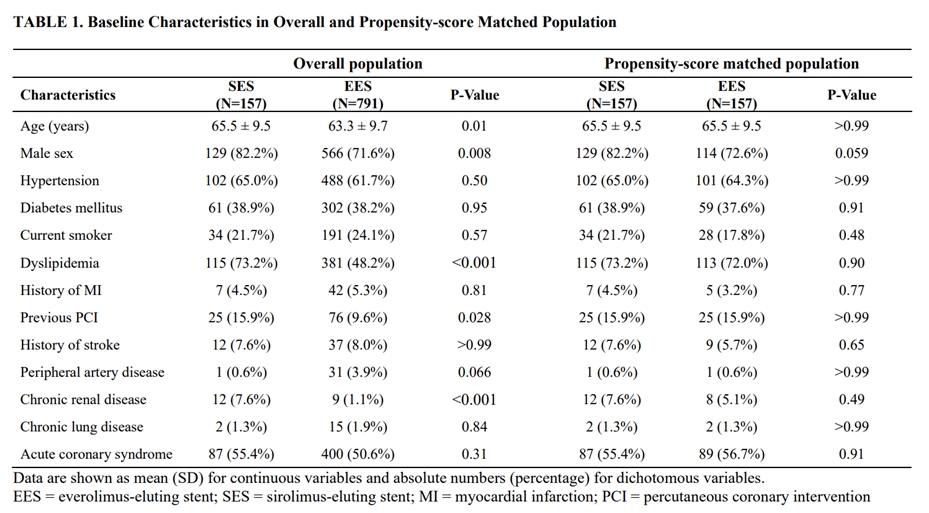
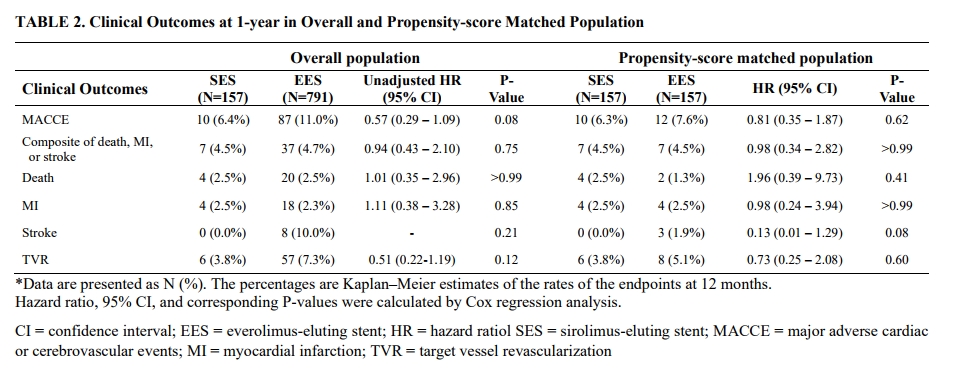
A total of 157 consecutive patients who implanted SES with biodegradable polymer for ULMCA stenosis between March 2014 to July 2021 were prospectively enrolled in the PRECOMBAT-5 registry. Clinical outcomes were compared with the 791 patients of PRECOMBAT-2 and 3 study who implanted everolimus-eluting stent (EES) for ULMCA stenosis. Differences in clinical characteristics between groups were adjusted using propensity-score matching. The primary outcome was the 1-year incidence rates of major adverse cardiac or cerebrovascular events (MACCE), including death, myocardial infarction (MI), stroke, or target vessel revascularization (TVR).
Results
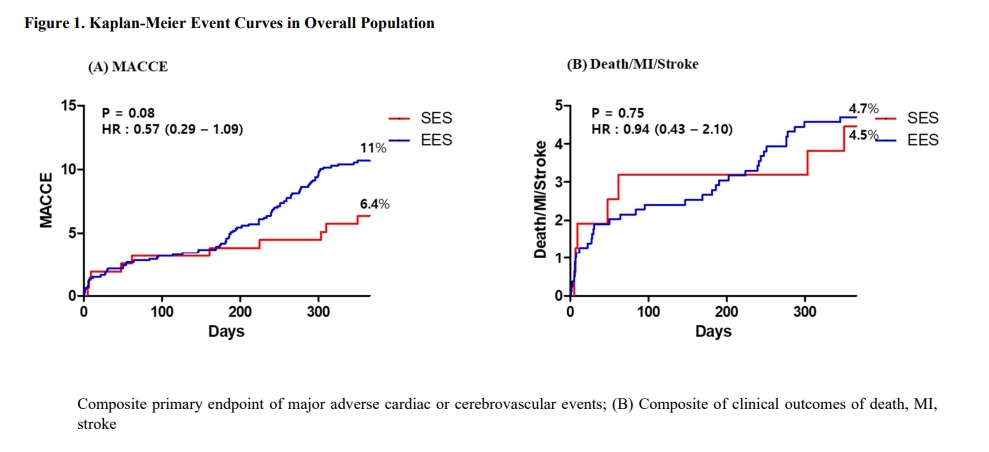
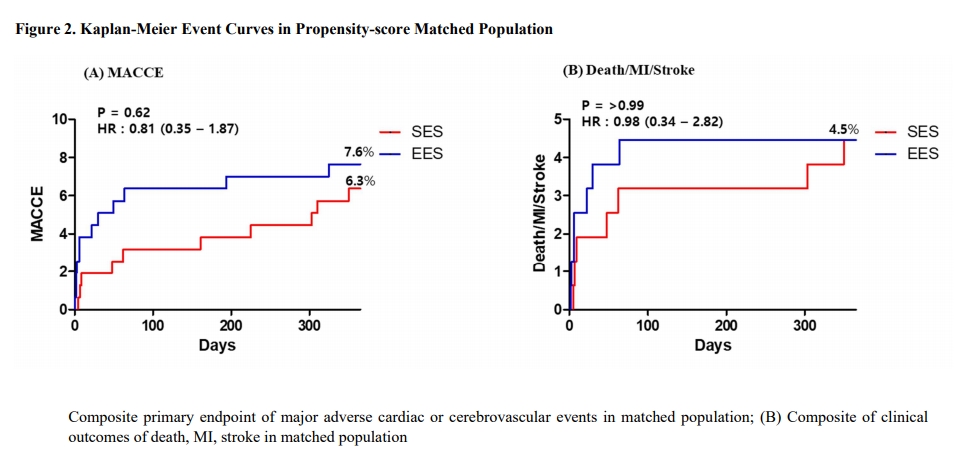
The 1-year incidence of MACCE were comparable between SES and EES group in overall (6.4% vs. 11.0%; Hazard ratio [HR] 0.57; 95% confidence interval [CI] 0.29-1.09; p=0.08) and propensity-score matched population (6.3% vs. 7.6%; HR 0.81; 95% CI 0.35-1.87; p=0.62). The composite of death, MI, or stroke also did not differ between SES and EES in overall (4.5% vs. 4.7%; HR 0.94; 95% CI 0.43-2.10; p=0.80) and propensity-score matched population (4.5% vs. 4.5%; HR 0.98; 95% CI 0.34-2.82; p>0.99).
Conclusion
In the multi-center, prospective, real-world data,PRECOMBAT-5 registry, SES with biodegradable polymer showed a comparable 1-year incidence rate of MACCE, and compositeof death, MI, or stroke for ULMCA PCI with second-generation EES.