Lots of interesting abstracts and cases were submitted for TCTAP 2022. Below are the accepted ones after a thorough review by our official reviewers. Don’t miss the opportunity to expand your knowledge and interact with authors as well as virtual participants by sharing your opinion in the comment section!
TCTAP A-079
Comparison Between One Versus Multiple Brand Stents On In-stent Restenosis Events During Percutaneous Coronary Intervention (COMBINED PCI Study)
By Arvin Romero Yumul, Ariel Miranda, Roy Garrido
Presenter
Arvin Romero Yumul
Authors
Arvin Romero Yumul1, Ariel Miranda1, Roy Garrido1
Affiliation
Cardinal Santos Medical Center, Philippines1
View Study Report
TCTAP A-079
Stents (Bare-metal, Drug-eluting)
Comparison Between One Versus Multiple Brand Stents On In-stent Restenosis Events During Percutaneous Coronary Intervention (COMBINED PCI Study)
Arvin Romero Yumul1, Ariel Miranda1, Roy Garrido1
Cardinal Santos Medical Center, Philippines1
Background
In-stent restenosis (ISR) remains a major determinant of poor outcome after percutaneous coronary intervention (PCI) despite the advent of drug-eluting stents (DES). Presently, different brands of DES are approved for use with each one having its unique advantage over the other mainly in terms of ISR occurrence and target lesion revascularization (TLR). There is no specific guideline recommendation regarding which specific stent to use despite several head-to-head comparison studies. However, real world practice is not always limited by using a brand exclusive strategy during PCI and may involve combination of different brands.
Methods
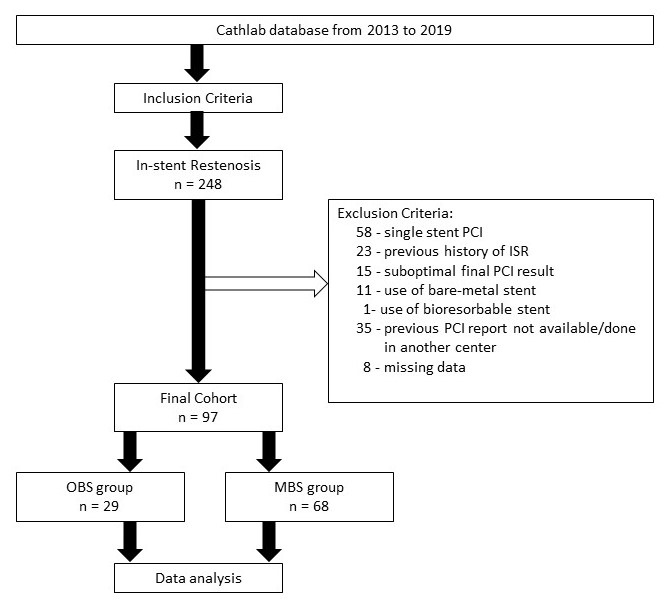
This was a retrospective observational cohort of patients who underwent PCI and later developed ISR events were divided into OBS group and MBS group. Clinical, angiographic, and procedural profiles were identified and the ISR and TLR events were compared between the groups.

Results
Of the 97 subjects, mean age was 62 years, majority were males, hypertensive, presenting with chronic stable angina with mean follow-up procedure duration of 16.3 months. The disease commonly involved 2 vessels without left main and requiring 3 stents per patient. The top 3 most used brands were Biomime (Meril, Gujarat, India), Xience Prime (Abbott Vascular, Chicago, IL, USA) and Resolute Integrity (Medtronic Inc, Santa Rosa, CA, USA) at 32%, 24%, and 23% respectively. In the OBS group, the most common type of stent used based on drug elution was Everolimus-eluting stent (EES) (65%), followed by Sirolimus-eluting stent (SES) (21%), Zotarolimus-eluting stent (ZES) (7%) and Paclitaxel-eluting stent (7%). In the MBS group, about half used 2-brand combination composing most commonly of 2 types of drug-eluting component namely EES+SES and SES+ZES, followed by 3 types of drug-eluting component of EES+SES+ZES. Majority of the ISR were severe with pattern IC being the most common. There was no significant difference on the (1) presentation on follow-up (p=0.514), (2) location, severity and pattern of ISR (p=0.272, 0.241, 0.110), and (3) target lesion revascularization (p=0.343) between the OBS and MBS group.
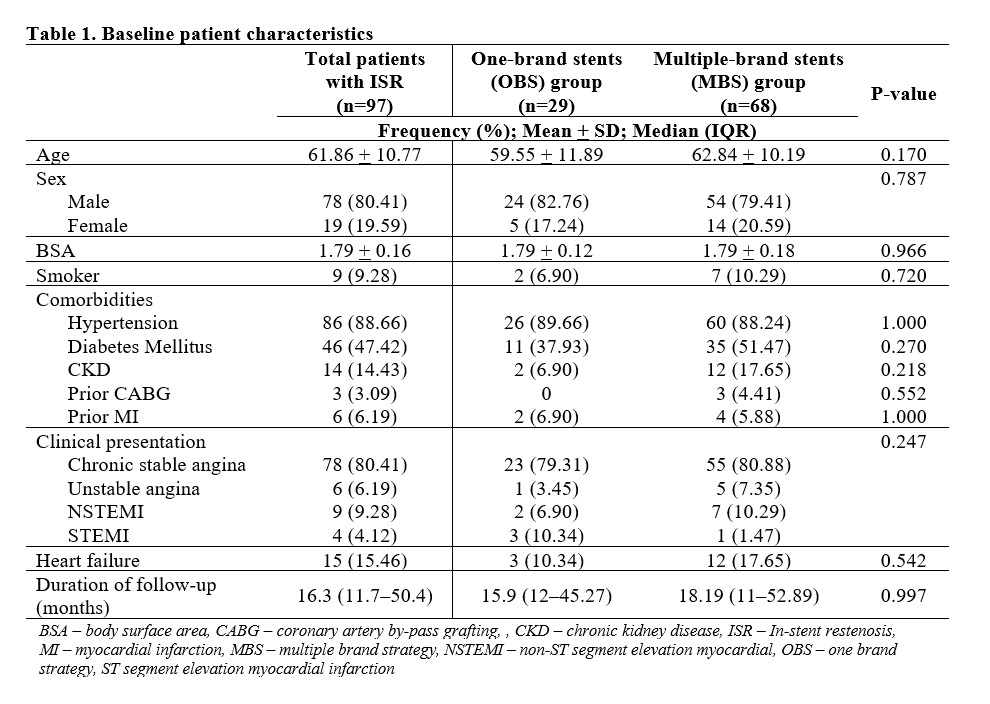
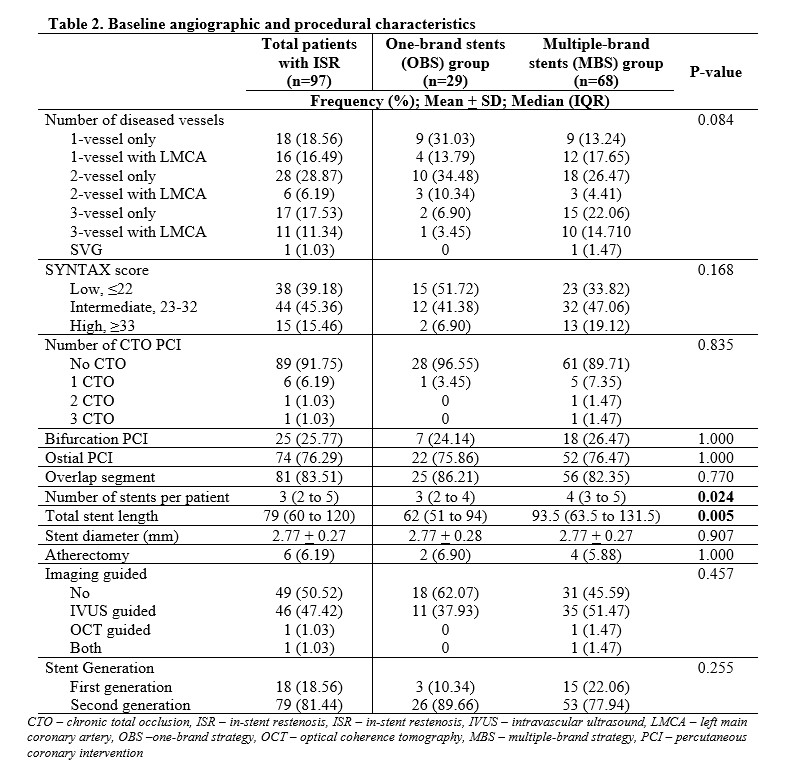
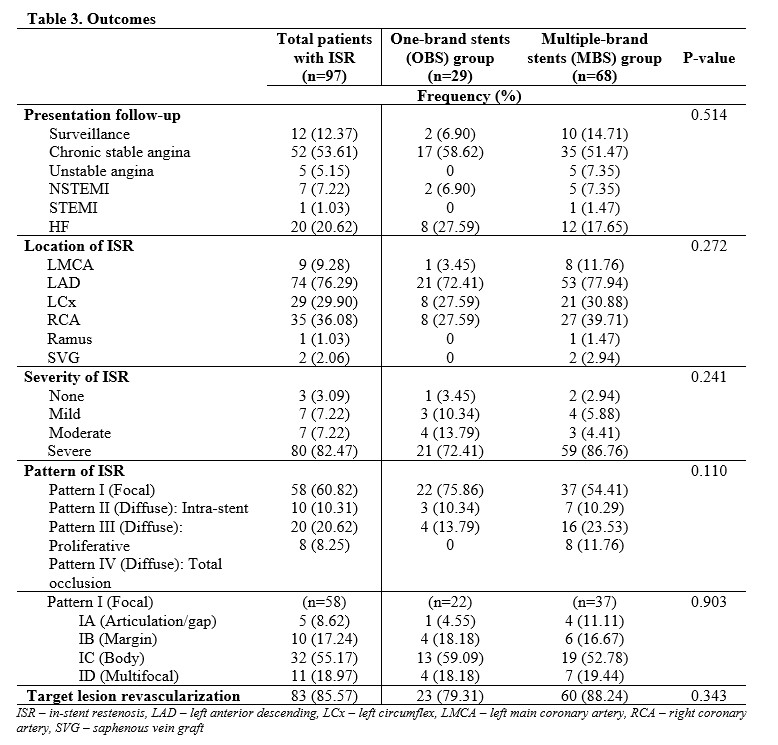



Conclusion
Despite differences in characteristics and features among several stent brands, these findings suggest that brand exclusivity does not affect the outcome of PCI procedures in terms of ISR and TLR events. Further studies are needed to establish a stronger comparison between the two strategies.


