Lots of interesting abstracts and cases were submitted for TCTAP 2022. Below are the accepted ones after a thorough review by our official reviewers. Don’t miss the opportunity to expand your knowledge and interact with authors as well as virtual participants by sharing your opinion in the comment section!
TCTAP A-017
Periprocedural Myocardial Infarction or Injury in Novel Low-Speed Following High-Speed and Conventional High-Speed Rotational Atherectomy Technique for Heavily Calcified Coronary Artery Lesions – The PRIMO-ROTA Pilot Study
By Mahesh Gurung, Anuruck Jeamanukoolkit, Wasant Soonfuang, Thotsaporn Morasert
Presenter
Mahesh Gurung
Authors
Mahesh Gurung1, Anuruck Jeamanukoolkit1, Wasant Soonfuang1, Thotsaporn Morasert2
Affiliation
Police General Hospital, Thailand1, Suratthani Hospital, Thailand2
View Study Report
TCTAP A-017
Adjunctive Procedures (Thrombectomy, Atherectomy, Special Balloons)
Periprocedural Myocardial Infarction or Injury in Novel Low-Speed Following High-Speed and Conventional High-Speed Rotational Atherectomy Technique for Heavily Calcified Coronary Artery Lesions – The PRIMO-ROTA Pilot Study
Mahesh Gurung1, Anuruck Jeamanukoolkit1, Wasant Soonfuang1, Thotsaporn Morasert2
Police General Hospital, Thailand1, Suratthani Hospital, Thailand2
Background
Rotational atherectomy (RA) or rotablation is an athero-ablative technology that enables percutaneous coronary intervention (PCI) of complex, calcified coronary lesions and is re-emerging with the advent of drug-eluting stents. The current best practices recommendations for RA with short ablation runs a ‘pecking’ motion, avoidance of sudden decelerations and the use of smaller burr sizes has led to substantial improvements in procedural safety and a reduced rate of associated complications. However, even in the era of the drug-eluting stents, the complications, but not limited to, such as death, peri-procedural myocardial infarction (PMI), dissection, perforation and slow-flow or no-reflow, still occur after RA, which have been linked to procedure-related morbidity and mortality. The incidence of RA related PMI range from 1 % to 19.8 %. Periprocedural myocardial infarction (PMI) leads to in-hospital and follow up major adverse cardiovascular events (MACE) when considering high risk patients and complex coronary lesion. Many studies have demonstrated that high burr speeds of more than 140 000 rpm cause significantly more activation of platelets, microcavitations and hemolysis. Therefore, recommendations on RA speed technique has also changed over time from conventional high-speed RA (HSRA), 180,000 to 200,000 rpm, to lower and safer range between 135,000 and 180,000 rpm. A recent study suggested that a new technique of using low-speed RA (LSRA) of 110,000 rpm following HSRA of 190,000 rpm have shown to attain larger lumen enlargement compared with conventional HSRA without significant occurrence of in-hospital complication between HSRA+LSRA and HSRA. This study aimed to compare the impact of two RA techniques (HSRA+LSRA vs. HSRA) on peri-procedural myocardial infarction or injury in patients with complex calcified coronary lesions and evaluate the immediate post-RA luminal gain between two RA techniques.
Methods
A prospective observational study was conducted that included all patients with complex coronary artery lesions who underwent LSRA after HRSA or conventional HSRA from January 2020 through March 2021 at Police General Hospital, Bangkok, Thailand.
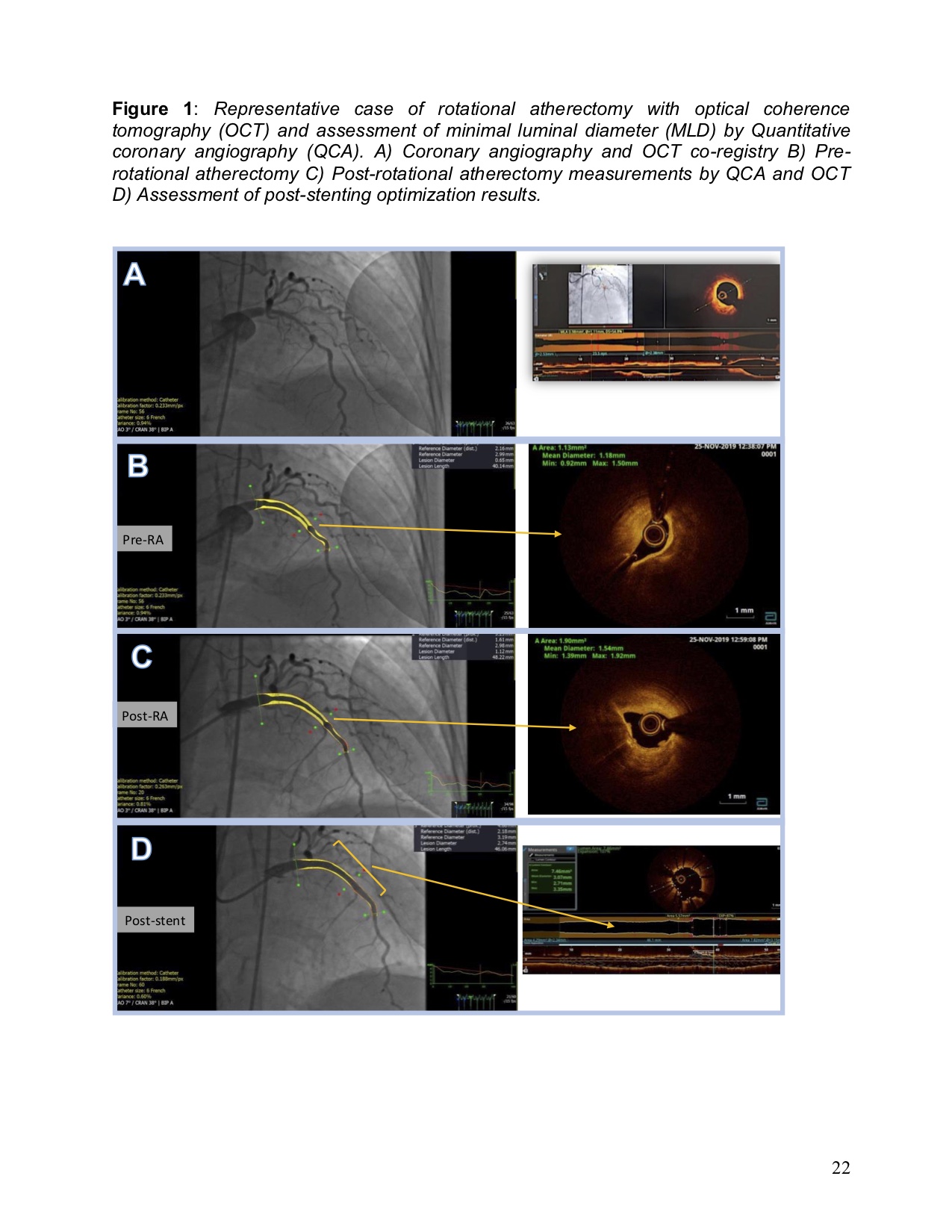

Results
21 patients were eligible for inclusion however 9 patients had to be excluded from this study due to incomplete data such as blood hs-cTnT levels and complete intracoronary images. 12 patients were included for the final analysis.
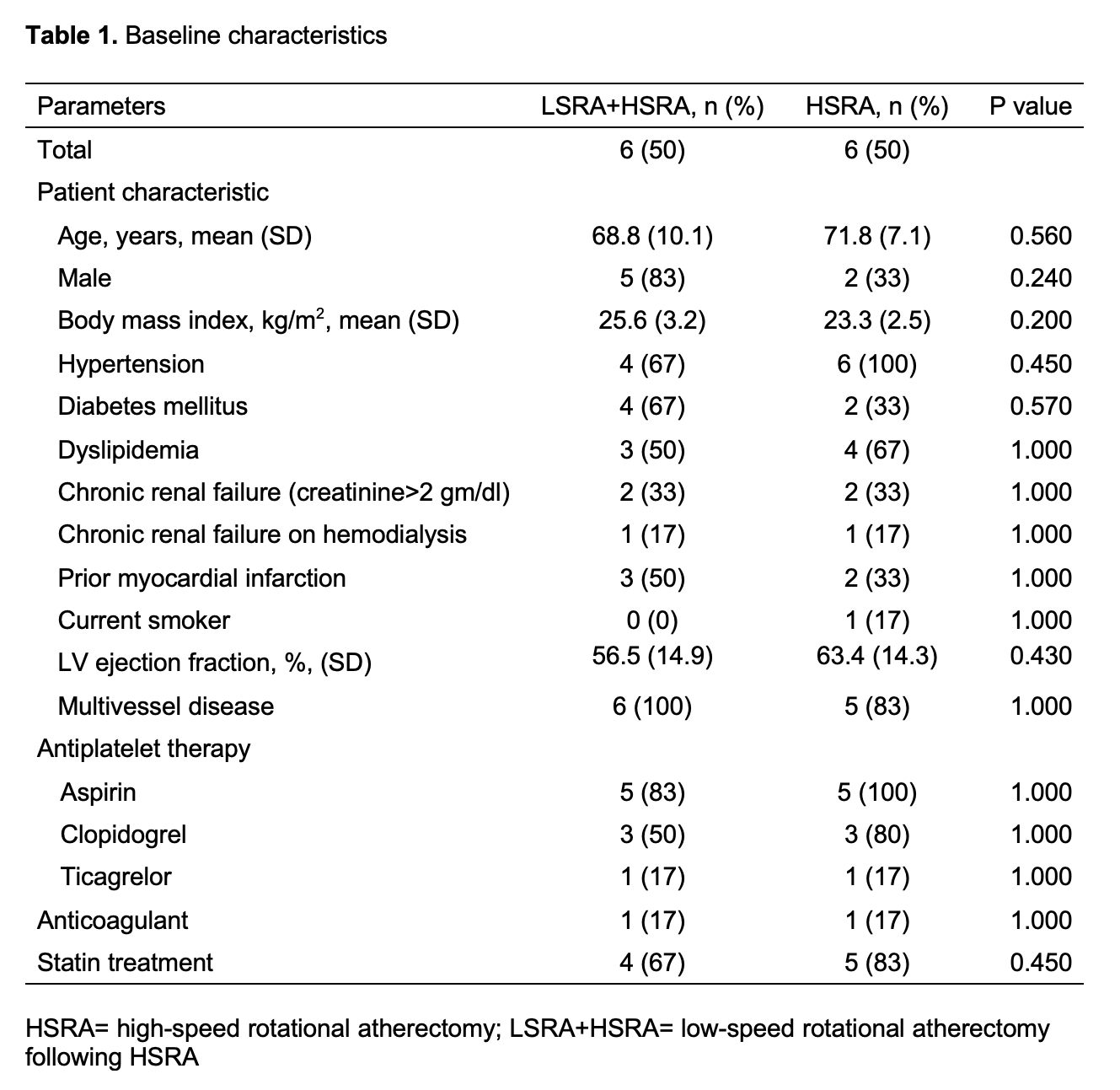
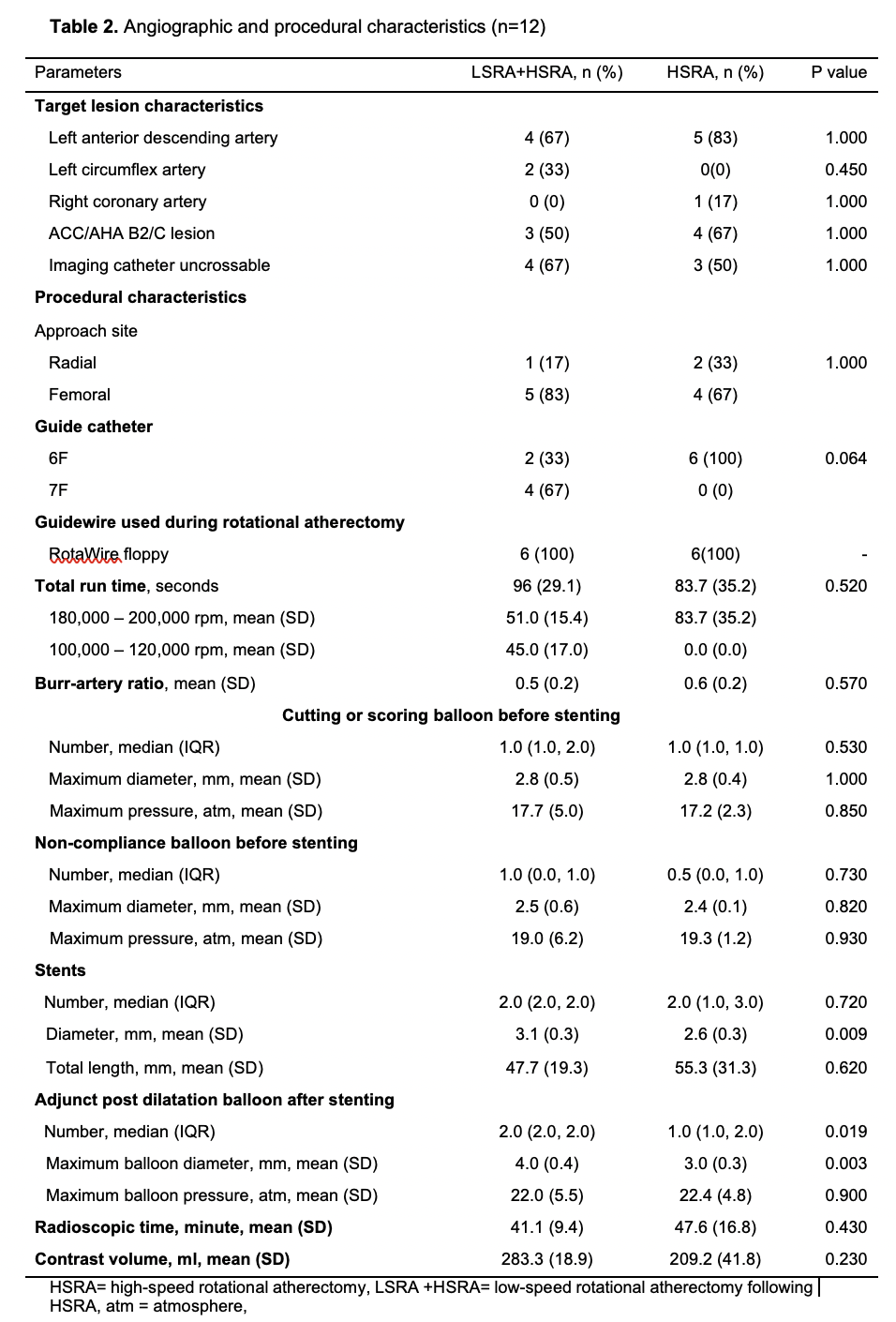
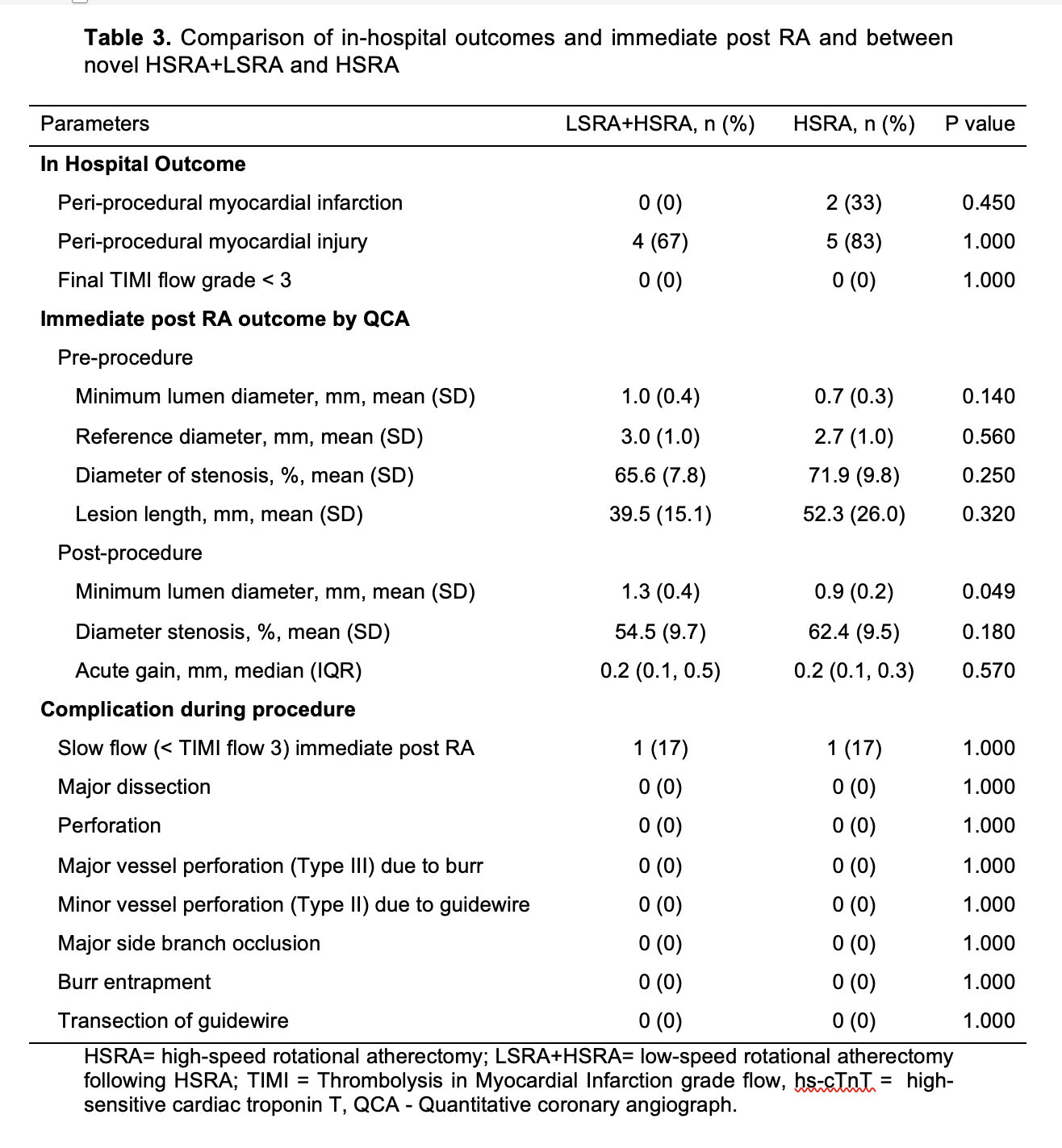
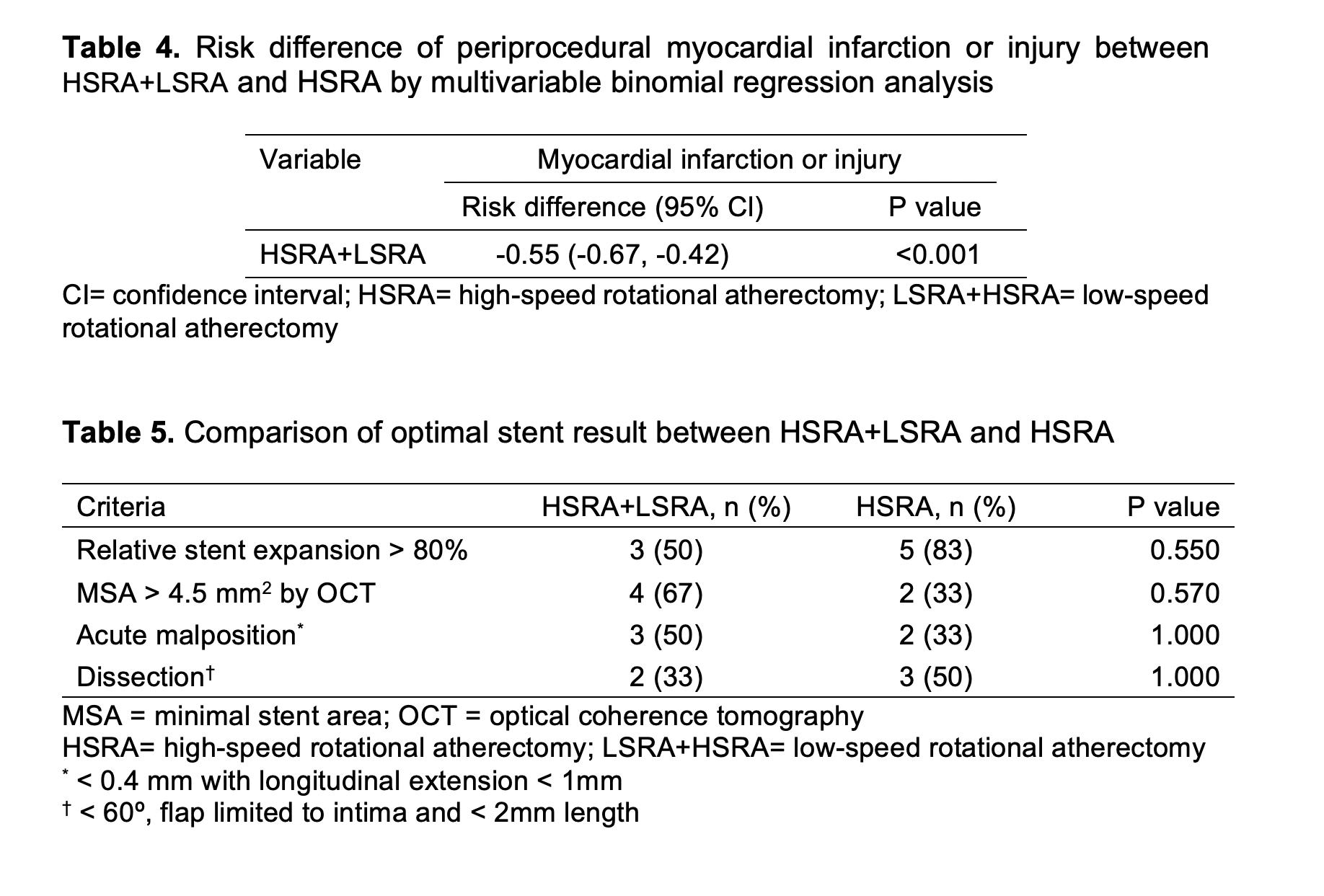
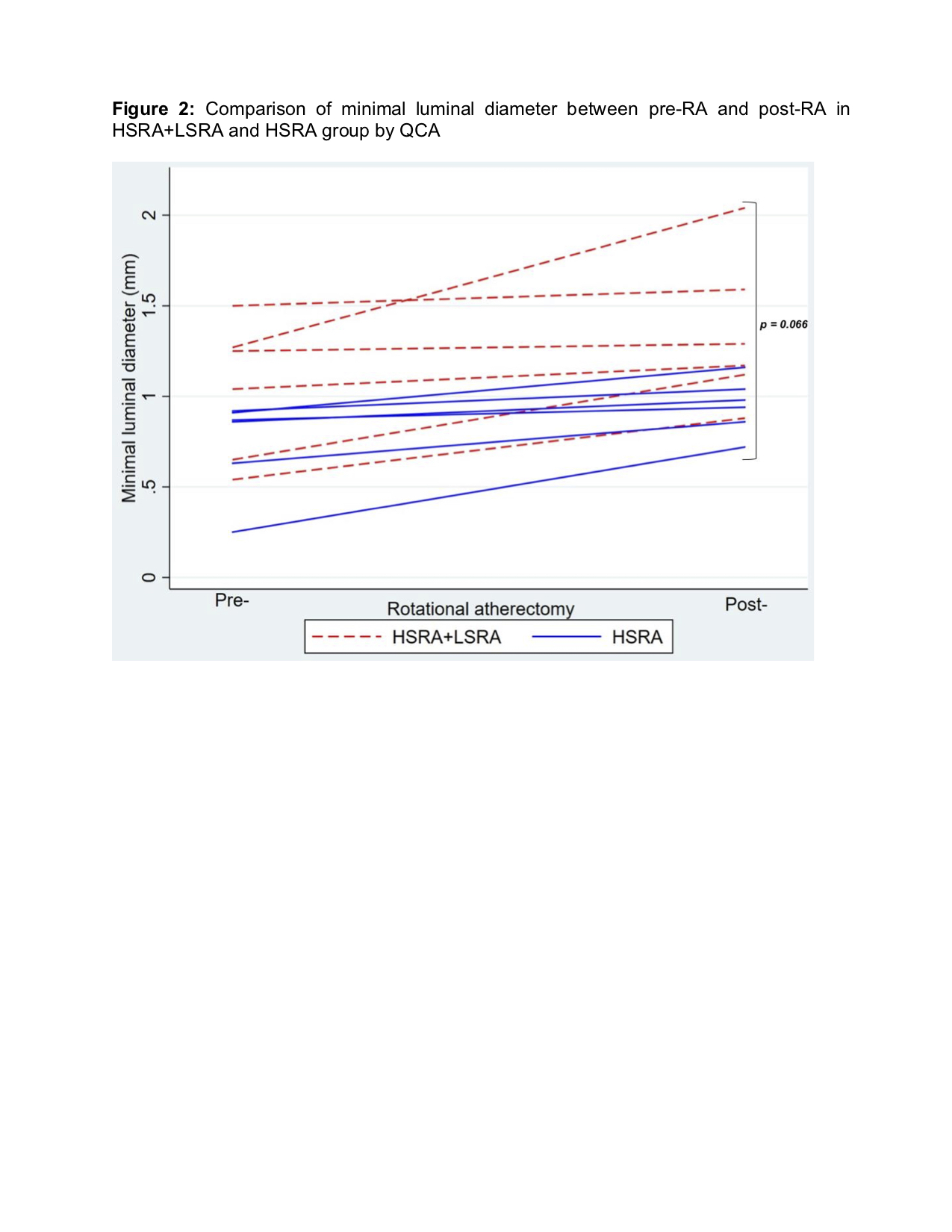





Conclusion
Periprocedural myocardial infarction or injury during rotational atherectomy is common however under addressed. Compared to the conventional high-speed rotational atherectomy, the novel technique of low-speed rotational atherectomy following high-speed rotational atherectomy significantly reduced the risk of periprocedural myocardial infarction or injury in heavily calcified long coronary artery lesions which could be attributed to possible lesser degree of activation of platelets, microcavitations and hemolysis in the latter group. There was trend towards larger post rotational atherectomy minimal luminal diameter in the new technique of low-speed rotational atherectomy following high-speed rotational atherectomy group. Further studies are needed to confirm these findings and its clinical impact on follow up major adverse cardiovascular events (MACE).


