New clinical trial data incorporating advances in drug therapy are shedding light on optimal antithrombotic therapy for patients with acute coronary syndrome (ACS) post-percutaneous coronary intervention (PCI).
 Deepak Bhatt, MD (Brigham and Women's Hospital, Boston, USA) highlighted antithrombotic therapy strategy updates for the post-PCI ACS patient group during a plenary session held at TCTAP 2021 Virtual.
Deepak Bhatt, MD (Brigham and Women's Hospital, Boston, USA) highlighted antithrombotic therapy strategy updates for the post-PCI ACS patient group during a plenary session held at TCTAP 2021 Virtual.
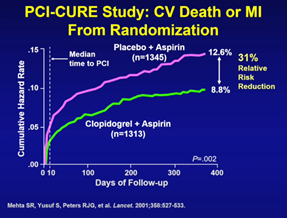
The PCI-CURE study, published nearly 20 years ago, established the role of “prolonged” one-year dual antiplatelet therapy (DAPT), demonstrating a significant reduction in the composite of cardiovascular death or myocardial infarction (MI) at 12-months compared to one-month DAPT (8.8% vs. 12.6%, HR 0.69).
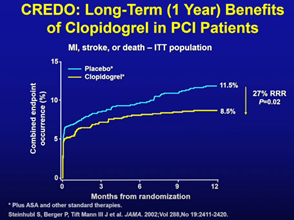
The CREDO trial, which compared one-year DAPT with one-month DAPT, also demonstrated a significant reduction in clinical events relating to MI, stroke, death in an elective post-PCI population (8.5% vs. 11.5%, HR 0.73, p=0.02).
The two trials ultimately demonstrated one-year DAPT was associated with large relative- and absolute-reduction of ischemic events
DAPT, however, posed a significant bleeding risk, particularly those pertaining to gastrointestinal bleeding.
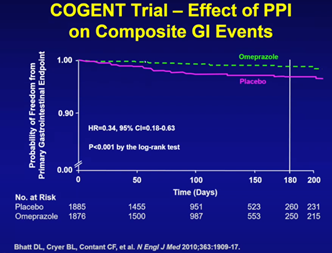 The COGENT trial demonstrated adding a proton-pump inhibitor (PPI) substantially mitigated gastrointestinal bleeding risk. Trial results showed adding PPI to DAPT either in the context of ACS or PCI significantly reduced gastrointestinal bleeding (omeprazole vs. placebo, HR 0.34, 95% CI 0.18-0.63, P<0.001).
The COGENT trial demonstrated adding a proton-pump inhibitor (PPI) substantially mitigated gastrointestinal bleeding risk. Trial results showed adding PPI to DAPT either in the context of ACS or PCI significantly reduced gastrointestinal bleeding (omeprazole vs. placebo, HR 0.34, 95% CI 0.18-0.63, P<0.001).
Several studies also showed extending DAPT beyond one-year prevented thrombotic consequences of plaque rupture, but came at the price of increased bleeding.
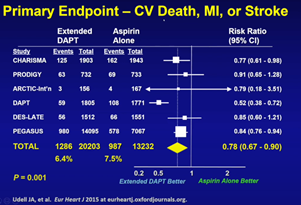
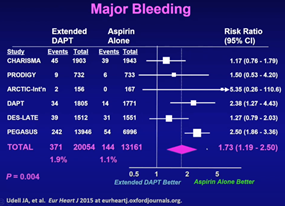
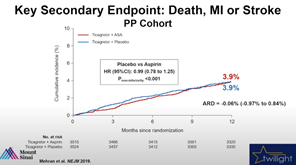
In a recent meta-analysis that pooled data from the PEGASUS, CHARISMA, PRODIGY, DES-LATE, DAPT, ARCTIC trials on ACS patients, extended DAPT significantly reduced the relative risk reduction regarding the composite of cardiovascular death, MI, or stroke compared to aspirin monotherapy with a 1.1 percent absolute reduction in risk (6.4% vs. 7.5%, HR 0.78, 95% CI 0.67-0.90).
Expectedly, investigators noted increased bleeding - a 0.8 percent absolute risk increase (1.9% vs 1.1%, HR 1.73 95% CI 1.19-2.50, P=0.004). This led the research team to conclude that extended DAPT provided a net clinical benefit in a broad, unselected population of ACS patients, albeit a small difference. Meanwhile, shorter DAPT also did not necessarily yield better outcomes for ACS, with the SMART-DATE randomized trial demonstrating higher risk of acute MI with six-month DAPT.
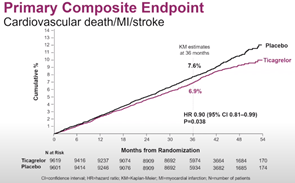
The THEMIS trial examined the combination of DAPT (ticagrelor+aspirin) compared to aspirin alone (aspirin+placebo) in diabetic patients with stable coronary artery disease.
Results showed a significant reduction in ischemic events such as cardiovascular death, MI, stroke at three-years, (6.9% vs. 7.6%, HR 0.90, 95% CI 0.81-0.99, P=0.038) but also increased bleeding events (2.2% vs. 1.0%, HR 2.32, 95% CI 1.82-2.94, p<0.001).
Compared to the aspirin arm, the ticagrelor+aspirin arm had a 10%p higher rate of permanent discontinuation due to bleeding or dyspnea. An on-treatment analysis showed the ticagrelor arm to be more robust, with a 19% relative risk reduction and a 1.2% absolute risk reduction (5.2% vs. 6.4%, HR 0.81, 95% CI 0.71-0.92, P=0.001).
“Based on the evidence, prolonged DAPT may be beneficial fora stable coronary artery disease population, particularly in a selected population who are able to tolerate the drug,” Bhatt said.
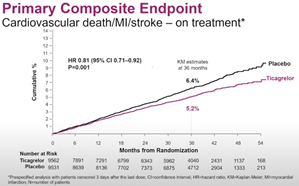
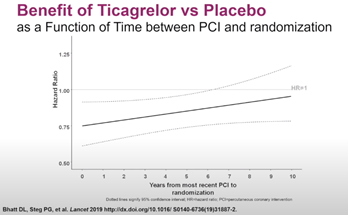
The THEMIS-PCI trial, which was a large pre-specified subgroup analysis from THEMIS trial, showed a significant reduction in MI, stroke and ST-segment elevation MI with ticagrelor, especially for those who remained on treatment. The benefits of adding ticagrelor to aspirin in THEMIS-PCI was extended as far as to 10 years after index PCI.
The FDA approved ticagrelor in June 2020 trials based on the THEMIS and THEMIS-PCI trials as a therapy to reduce the risk of first MI or stroke in high-risk CAD patients.
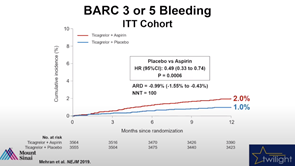
 Bhatt outlined the increasing support for abbreviating DAPT duration, highlighted by the results of the TWILIGHT trial. The study looked at high-risk patients in terms of high clinical and angiographic risk (excluding STEMI) after PCI.
Bhatt outlined the increasing support for abbreviating DAPT duration, highlighted by the results of the TWILIGHT trial. The study looked at high-risk patients in terms of high clinical and angiographic risk (excluding STEMI) after PCI.
Patients, after an initial run-in period of three-month DAPT of ticagrelor and aspirin, were randomized to DAPT or ticagrelor monotherapy (ticagrelor and placebo) for 12-months.
Results showed a 51 percent reduction in bleeding in the ticagrelor monotherapy arm (DAPT 2.0% vs. ticagrelor monotherapy 1.0%, HR 0.49, 95% CI 0.33- 0.74, P = 0.0006).
There was no significant difference in ischemic events such as death, MI and stroke over one-year follow-up.
Bhatt speculated that - considering TICO, TICO-STEMI, SMART-CHOICE, SMART-DAPT trials also support abbreviated DAPT-, an extended follow-up similar to THEMIS could prove potential benefit of abbreviated DAPT.
To settle the conundrum of DAPT duration and intensity associated with bleeding risk, Bhatt introduced several guiding tools for clinicians.
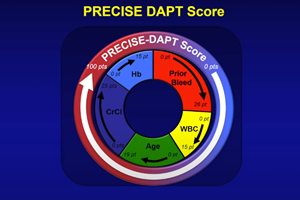
“The PRECISE-DAPT score, available as an online calculator, is a powerful tool for determining high bleeding risk (HBR) while other angiographic scores can also help determine complexity and suitability for abbreviated or extended DAPT,” he said.
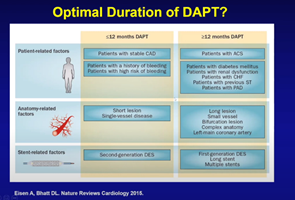
Bhatt also suggested amalgamating patient-related factors with anatomical- and stent- related factors to determine whether extended DAPT (>12 months) is suitable for a particular patient.
"In ACS patients, we still need at least 12-months of DAPT if the patient has no bleeding,” Bhatt said. “But if patients have a high bleeding risk, the physician can abbreviate to 3-month DAPT. At that point, ticagrelor is preferred and clopidogrel monotherapy serves as an option in selected patients. If patients have a history of MI, complex coronary stenting or disease, the physician should consider extended DAPT spanning beyond one-year,” he said.
Edited by

Hanbit Park , MD
GangNeung Asan Hospital, Korea (Republic of)


