Lots of interesting abstracts and cases were submitted for TCTAP 2021 Virtual. Below are accepted ones after thoroughly reviewed by our official reviewers. Don’t miss the opportunity to explore your knowledge and interact with authors as well as virtual participants by sharing your opinion!
TCTAP A-030
Presenter
Hendy Bhaskara Perdana Putra
Authors
Hendy Bhaskara Perdana Putra1, Quri Meihaerani Savitri2, Wally Wahyu Mukhammad3, Atiyatum Billah4, Alan Dharmasaputra3, Ragil Nur Rosyadi1
Affiliation
Dr. Ramelan Navy Hospital Surabaya, Indonesia1, West Nusa Tenggara Eye Hospital, Mataram, Indonesia2, Hang Tuah University, Indonesia3, Hang Tuah University, Indonesia, Indonesia4
View Study Report
TCTAP A-030
Drug-Eluting Balloons
Drug Coated Balloon Versus Drug-eluting Stent for In-stent Restenosis After Drug-eluting Stent Implantation: A Meta-analysis
Hendy Bhaskara Perdana Putra1, Quri Meihaerani Savitri2, Wally Wahyu Mukhammad3, Atiyatum Billah4, Alan Dharmasaputra3, Ragil Nur Rosyadi1
Dr. Ramelan Navy Hospital Surabaya, Indonesia1, West Nusa Tenggara Eye Hospital, Mataram, Indonesia2, Hang Tuah University, Indonesia3, Hang Tuah University, Indonesia, Indonesia4
Background
Drug-eluting stents (DES) have shown great improvement in reducing in-stent restenosis (ISR). However, the incidence of ISR was still a problem after DES implantation. Drug-Coated Balloon (DCB) has been accepted as a treatment for ISR-DES. Several studies have been conducted to assess the benefits of DCB for dealing with ISR-DES. However, the results remain conflicting.
Methods
The SURABAYA (reSUlt of dRug coAted Balloon AngioplastY versus drug-eluting stent for in-stent restenosis After drug-eluting stent implantation) Study is a meta-analysis to compare safety and efficacy of DCB treating ISR-DES. SURABAYA Study performed a systematic literature search from several electronic databases. The inclusion criteria were studies comparing DCB and DES to treat ISR-DES, both (Randomized Control Trial) RCT and observational study were acceptable. The exclusion criteria were studies comparing DCB and DES to treat ISR-BMS. The primary safety endpoint was cardiac death and the primary efficacy endpoint was target vascular revascularization (TVR). Secondary endpoints included myocardial infarction (MI), definite stent thrombosis, and major cardiovascular adverse events (MACE). Risk ratios with 95% confidence intervals (CIs) was calculated with the appropriate fixed or random-effects models.
Results
A total of 4 RCT and 4 observational study were selected, with 1.610 patients were pooled in our analysis. Comparing to DES, DCB had a similar outcome in primary safety end point, cardiac death (RR=1.07 [95% CI, 0.48-2.35], p=0.87). However, DCB had higher risk of primary efficacy end point, TVR (RR=1.30 [95% CI, 1.01-1.68], p=0.04). myocardial infarction (RR=0.79 [95% CI, 0.41-1.52], p=0.48), stent thrombosis (RR=0.50 [95% CI, 0.09-2.65], p=0.42), and MACE (RR=1.21 [95% CI, 0.93-1.59], p=0.15).
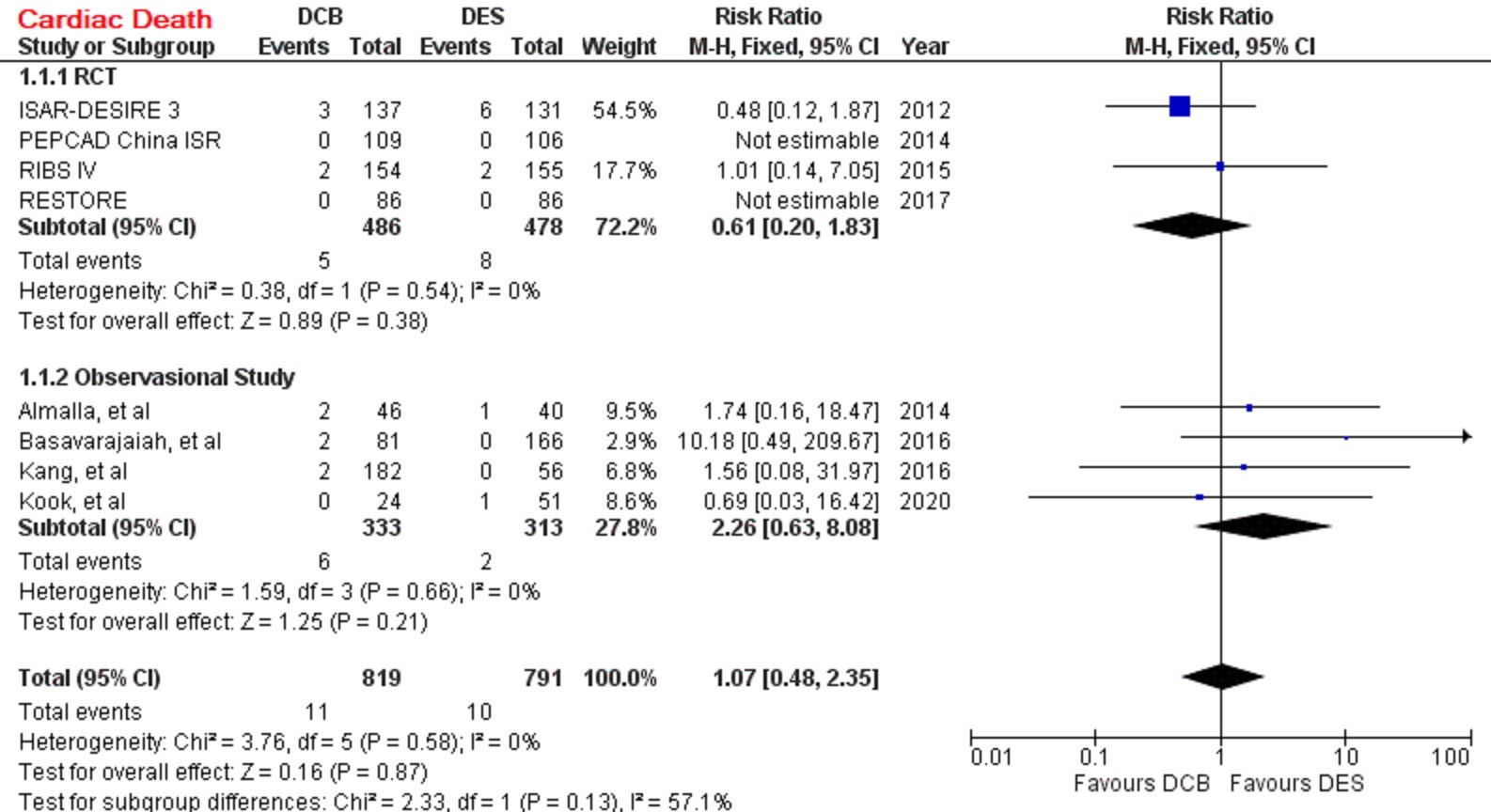
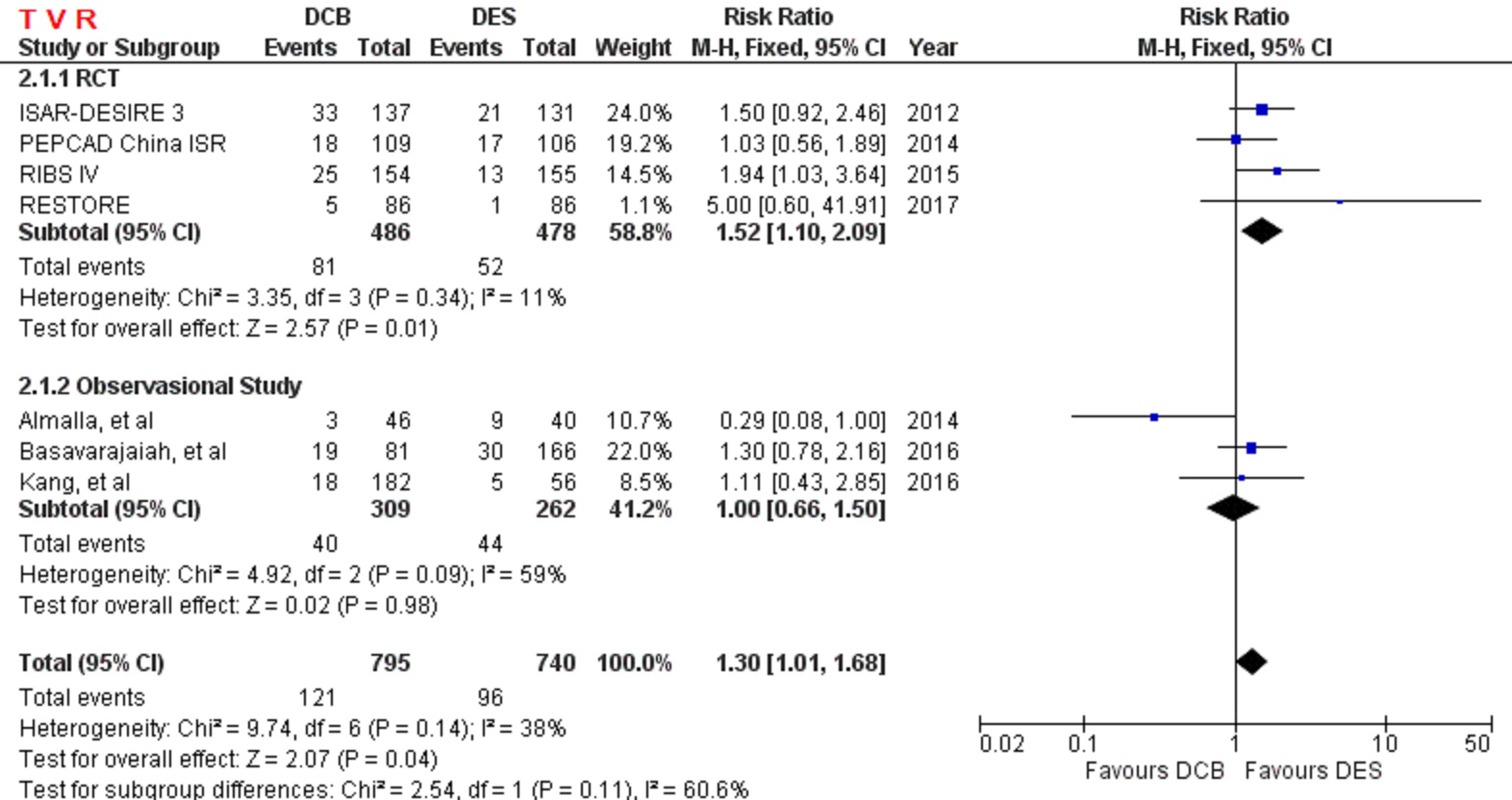


Conclusion
DCB is as safe as DES in treating ISR-DES. DCB also has comparable outcomes in myocardial infarction, stent thrombosis, and MACE. However, DCB is less effective than DES to prevent an incident of TVR.


