
William F. Fearon
Stanford University, USA
In the Ī░Late-Breaking Clinical Trials 2025Ī▒ session at TCTAP 2025, Dr. William F. Fearon (Stanford University, USA) presented the final 5-year results of the FAME 3 trial, showing that fractional flow reserve (FFR)-guided PCI delivers comparable long-term outcomes to coronary artery bypass grafting (CABG) in patients with three-vessel coronary artery disease.
CABG has long been considered the gold standard for multivessel coronary artery disease, with previous studies consistently demonstrating its long-term superiority over PCI. But according to Fearon, most of that data is outdated. FAME 3 trial was designed to reflect contemporary clinical practice, incorporating advanced stent technologies, physiology-guided PCI, and optimal medical therapy.
The FAME 3 trial enrolled 1,500 patients across 48 centers worldwide, randomizing them to receive either FFR-guided PCI with contemporary drug-eluting stents or angiography-guided CABG. Importantly, both groups received optimal medical therapy, with high rates of statin, antiplatelet, beta-blocker, and RAS inhibitor use.
At five years, the composite endpoint of death, stroke, or MI occurred in 16% of PCI patients and 14% of CABG patients (HR 1.16, 95% CI 0.89–1.52; p=0.27) (Figure). Landmark analysis indicated that the early advantages of CABG did not extend beyond the first year. Mortality was identical in both arms at 7.2%. While the stroke rate was numerically lower with PCI (1.9% vs. 3%), MI (8.2% vs. 5.3%) and repeat revascularization (15.6% vs. 7.8%) occurred more frequently in the PCI group (Table).
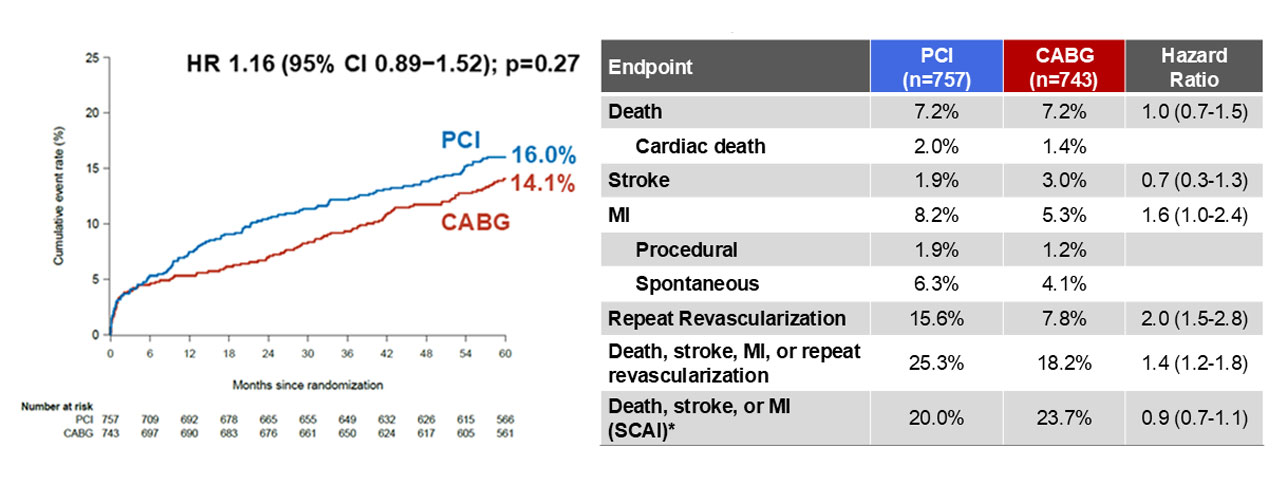
Ī░Despite higher rates of MI and repeat revascularization, we found no difference in the hard endpoint of death, stroke, or MI at 5 years between FFR-guided PCI and CABG,Ī▒ Dr. Fearon said. Ī░This marks a notable shift from previous trials. There was no late accrual of benefit with CABG after the first year.Ī▒
Subgroup analysis based on SYNTAX score revealed significant interaction: patients with low (<23) SYNTAX score had better outcomes with PCI, whereas those with intermediate SYNTAX score (23–32) had better outcomes with CABG.
Comparing the data to the SYNTAX trial, Dr. Fearon highlighted the substantial progress in PCI outcomes. In SYNTAX trial, the 5-year rate of death, stroke, or MI following PCI was 22%, far higher than the 16% we observed in FAME 3 trial. He explained that the narrowing gap in outcomes at 5 years is Ī░likely due to improved stent technology, the routine application of FFR-guided PCI, and greater adherence to guideline-directed medical therapy.Ī▒
The FAME 3 trial demonstrated that, in the context of contemporary PCI techniques and medical therapy, FFR-guided PCI can yield long-term outcomes comparable to CABG in patients with three-vessel disease. Dr. Fearon emphasized that the FAME 3 trial provides contemporary data regarding outcomes after FFR-guided PCI using current-generation DES compared with CABG in patients with three-vessel coronary artery disease, which can facilitate improved shared decision-making between physicians and patients.
Late-Breaking Clinical Trials 2025
Thursday, April 24, 4:30 PM-5:50 PM
Presentation Room 1, Level 1
Edited by

Sangwoo Park, MD
Ulsan University Hospital, Korea (Republic of)

