
Min Soo Cho
Asan Medical Center, Korea (Republic of)
In patients with coronary artery disease (CAD), the prevalence of atrial fibrillation (AF) is significantly high. However, optimal antithrombotic therapy for managing both conditions is challenging. Previous clinical trials suggested the preferred use of direct oral anticoagulants (DOAC) and dual antithrombotic therapy (DOAC + clopidogrel), followed by the short-term use of triple therapy (DOAC + dual antiplatelet). However, beyond the first year, there remains limited evidence regarding the best long-term management strategy, especially in patients with stable CAD and AF.
The EPIC-CAD trial, presented at ESC 2024 and published in the New England Journal of Medicine, tested the hypothesis that standard-dose Edoxaban monotherapy would be superior to dual antithrombotic therapy (Edoxaban plus a single antiplatelet agent) in terms of the primary net clinical outcome in patients with high-risk AF (CHA2DS2-VASc score Ī├2) and stable CAD (for those with prior revascularization: Ī├12 months for acute coronary syndrome and Ī├6 months for chronic CAD; for those on medical therapy only: coronary artery stenosis Ī├50%). The primary endpoint was a composite of all-cause mortality, stroke, systemic embolism, myocardial infarction, unplanned revascularization, and major or clinically relevant non-major bleeding at 12 months. (Figure 1)
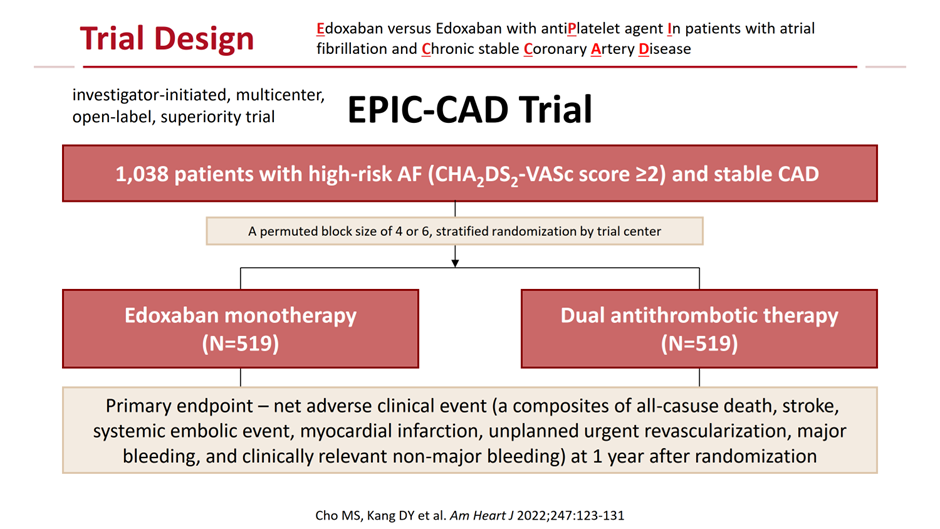
1,040 patients were randomized 1:1 to receive either Edoxaban monotherapy (60 mg or 30 mg based on dose-reduction criteria) or dual antithrombotic therapy. The mean age was 72, 22% were women; the mean CHA2DS2-VASc and HAS-BLED scores were 4.3 and 2.1, and 66% had undergone previous revascularization. At 12 months, Edoxaban monotherapy significantly reduced the risk of the primary endpoint by 56% compared to dual antithrombotic therapy (6.8% vs. 16.2%; hazard ratio [HR] 0.44; 95% confidence interval [CI] 0.30-0.65; p<0.001). This reduction was primarily driven by a 66% lower risk of major or clinically relevant non-major bleeding (4.7% vs. 14.2%; HR 0.34; 95% CI 0.22-0.53). Importantly, no significant difference was observed in major ischemic events between the two groups (1.6% vs. 1.8%; HR 1.23; 95% CI 0.48-3.10) (Figure 2).
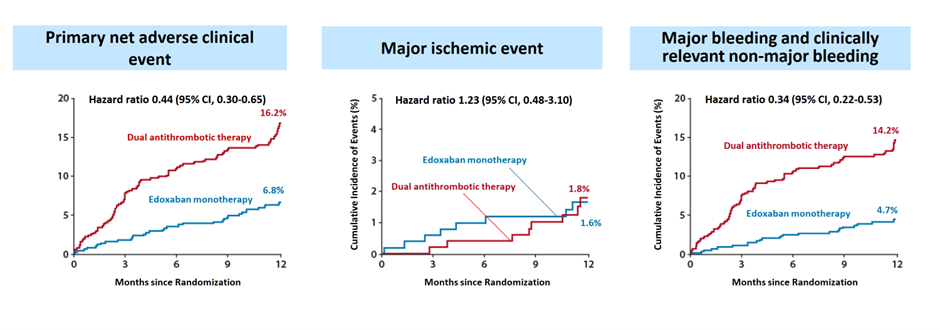
The findings of the EPIC-CAD trial suggest that standard-dose Edoxaban monotherapy is safer and equally effective as dual antithrombotic therapy for long-term treatment in high-risk AF patients with stable CAD, particularly by significantly reducing bleeding risk without increasing ischemic events. These results align with previous data from the AFIRE trial, which demonstrated the non-inferiority of low-dose rivaroxaban monotherapy (15 mg or 10 mg) compared to dual antithrombotic therapy in a similar patient population. By employing a globally approved standard dosing regimen, the EPIC-CAD trial provides robust evidence supporting current guidelines advocating for anticoagulant monotherapy in patients with AF and stable CAD.
Best and Novel Medical Therapy in Interventional Cardiology: What Are New in 2025?
Thursday, April 24, 3:30 PM - 5:00 PM
Presentation Room 2, Level 1
Edited by

Hansu Park, MD
Asan Medical Center, Korea (Republic of)

