Lots of interesting abstracts and cases were submitted for TCTAP 2025. Below are the accepted ones after a thorough review by our official reviewers. Don’t miss the opportunity to expand your knowledge and interact with authors as well as virtual participants by sharing your opinion in the comment section!
TCTAP C-206
Gadolinium-Guided TAVI: A Contrast Alternative for High-Risk Patients With Severe Aortic Stenosis
By Ahmed Alhaydhal, Hussein Alamri, Abdulrahman Al-Moghairi
Presenter
Ahmed Alhaydhal
Authors
Ahmed Alhaydhal1, Hussein Alamri1, Abdulrahman Al-Moghairi1
Affiliation
Prince Sultan Cardiac Center, Saudi Arabia1,
View Study Report
TCTAP C-206
Structural - Aortic Valve Intervention - Complex TAVR
Gadolinium-Guided TAVI: A Contrast Alternative for High-Risk Patients With Severe Aortic Stenosis
Ahmed Alhaydhal1, Hussein Alamri1, Abdulrahman Al-Moghairi1
Prince Sultan Cardiac Center, Saudi Arabia1,
Clinical Information
Patient initials or Identifier Number
Relevant Clinical History and Physical Exam
A 90 years old male was admitted for evaluation and management of severe symptomatic AS. He presented with New York Heart Association (NYHA) class III dyspnea and recurrent exertional syncope. His past medical history included insulin requiring diabetes mellitus (IRDM), hyperlipidemia, hypertension, benign prostate hyperplasia and chronic kidney disease
CVS: S1, S2 normal , no murmurs or added sounds, JVP not raised and no lower limbs edemaCHEST:clear with normal air entry
ABD:soft and no organomegally
CNS:intact
Groin : Intact with no bleeding and good peripheral pulses
Relevant Test Results Prior to Catheterization
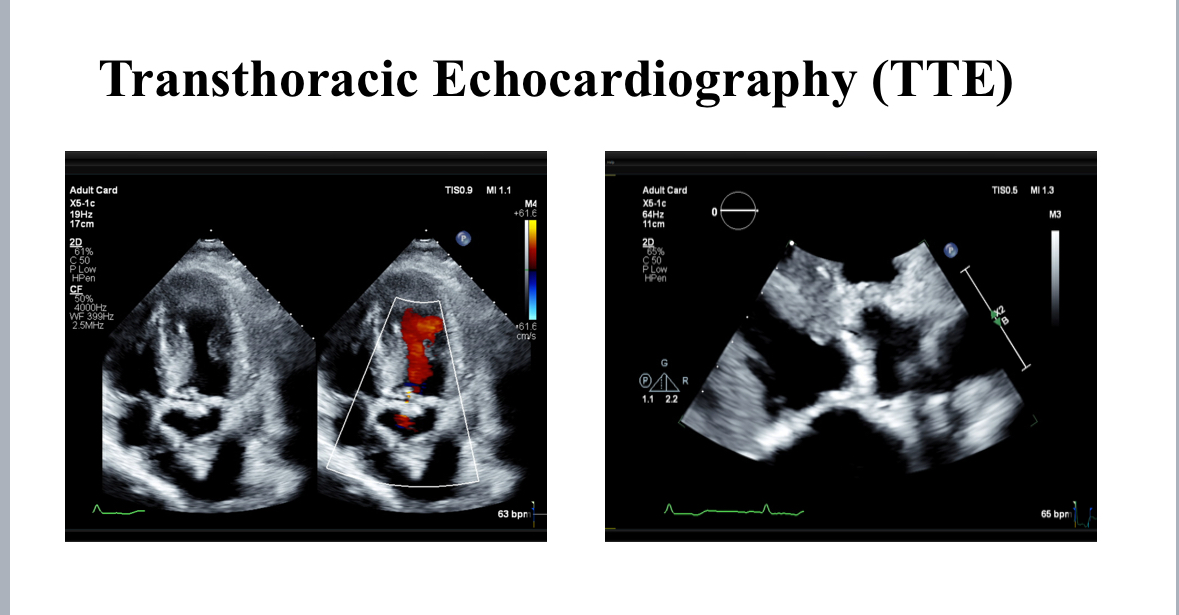
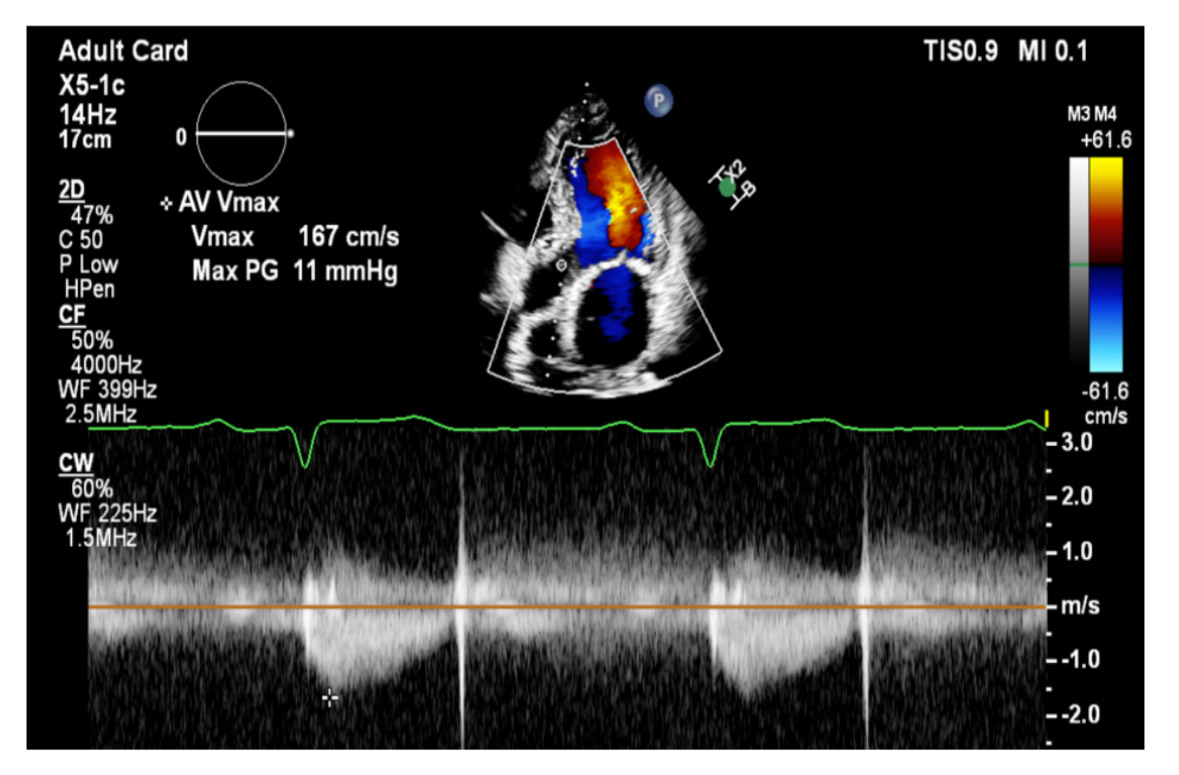
creatinine 150 mcmol/L, estimated glomerular filtration rate: 38 mL/min/1.73m2, creatinine clearance: 28 mL/ min). In 1998, he underwent CABG with LIMA to LAD, and SVG to D1and OM1. In 2020, he underwent PCI and stent to LIMA-LAD, RCA and SVG to OM1.Transthoracic echocardiography showed an ejection fraction of 55%, severely calcified tricuspid aortic valve, aortic valve area of 0.9 cm2, peak gradient of -- mmHg, mean gradient of – mmHg, Vmax – and dimensionless index of 0.22. ECG showed normal sinus rhythm and a heart rate of 55 beat per minute.
Relevant Catheterization Findings
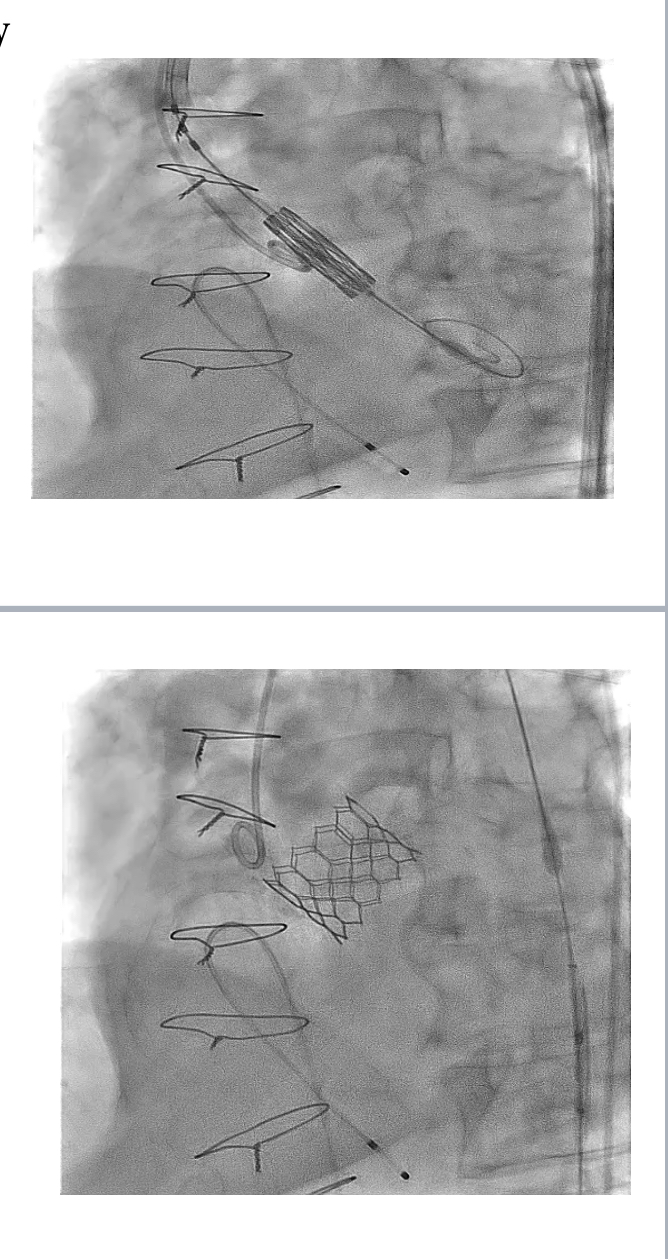
Pre-TAVI evaluation included a zero-contrast (dry) cardiac CT, TEE and femoral doppler ultrasound. Zero-contrast CT and TEE showed an AVA area of 617 mm2, perimeter of 89 mm, LM hight 17 mm, RCA hight 14 mm, mean sinus of valsalva and sino-tubular junction of 34 mm and 33 mm respectively. The femoral arteries were heavily calcified, with modertate torousisty and a minimal lumen diameter of 8.4 mm.After informed consent, the TAVI procedure was performed in the Hybrid Cathlab following the standard transfemoral approach. The femoral sheathes were inserted under flouroscopy and US guidance. The aortic valve was crossed and a Confida wire was palced in the left ventricle. The Pigtail was placed in the noncoronary cusp. A baseline aortogram was performed using Gadobutrol via automated injector. A 29 Sapien 3 ultra-valve (Edwards Lifesciences, Irvine, California) was deplyed after Gadobutrol aortogram to confirm the position.
Interventional Management
Procedural Step
The pre-TAVI evaluation included zero-contrast (dry) cardiac CT, transesophageal echocardiography (TEE), and femoral Doppler ultrasound. Imaging revealed a severely calcified tricuspid aortic valve with an aortic valve area of 617 mm², a perimeter of 89 mm, a left main coronary artery height of 17 mm, and a right coronary artery height of 14 mm. The mean sinus of Valsalva and sino-tubular junction measured 34 mm and 33 mm, respectively. The femoral arteries exhibited heavy calcification with moderate tortuosity and a minimal lumen diameter of 8.4 mm.
Following informed consent, the TAVI procedure was performed in the hybrid catheterization lab via a standard transfemoral approach. Femoral sheaths were inserted under fluoroscopic and ultrasound guidance, and the aortic valve was crossed with a Confida wire placed in the left ventricle. A pigtail catheter was positioned in the non-coronary cusp, and a baseline aortogram was performed using Gadobutrol administered via an automated injector. A 29 mm Sapien 3 Ultra valve (Edwards Lifesciences, Irvine, California) was deployed after confirming its position with a Gadobutrol aortogram. The image quality was comparable to iodinated contrast media (ICM), and the procedure was successfully completed without any adverse reactions to Gadobutrol.
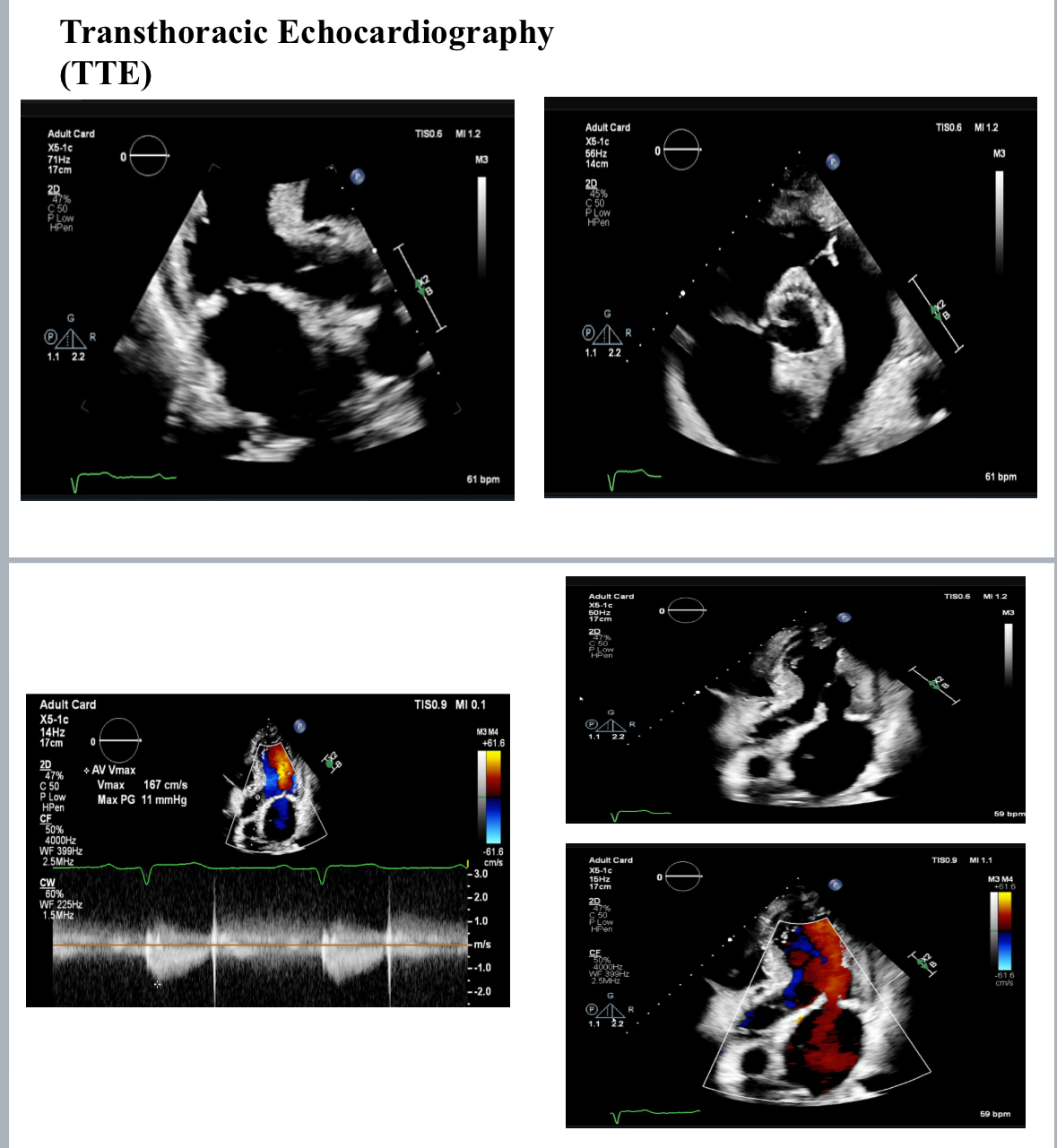
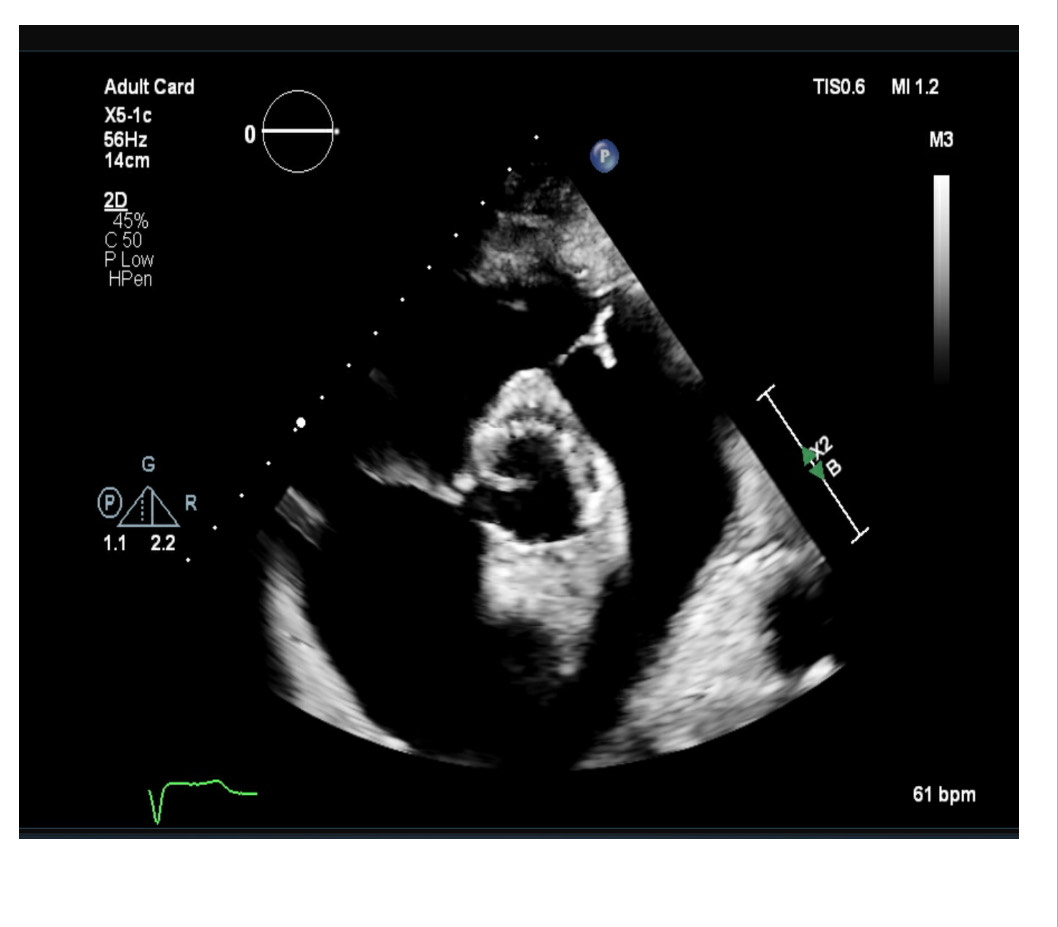
Post-TAVI aortography confirmed proper valve positioning with only a trivial paravalvular leak (PVL). The access sites were successfully closed using ProGlide and Angio-Seal devices. A total of 50 cc of Gadobutrol was used. Pre-discharge transthoracic echocardiography (TTE) confirmed good transcatheter heart valve (THV) positioning, no PVL, and a peak gradient of 6 mmHg. Renal function tests performed one week post-procedure showed no deterioration, with a creatinine level of 156 µmol/L.
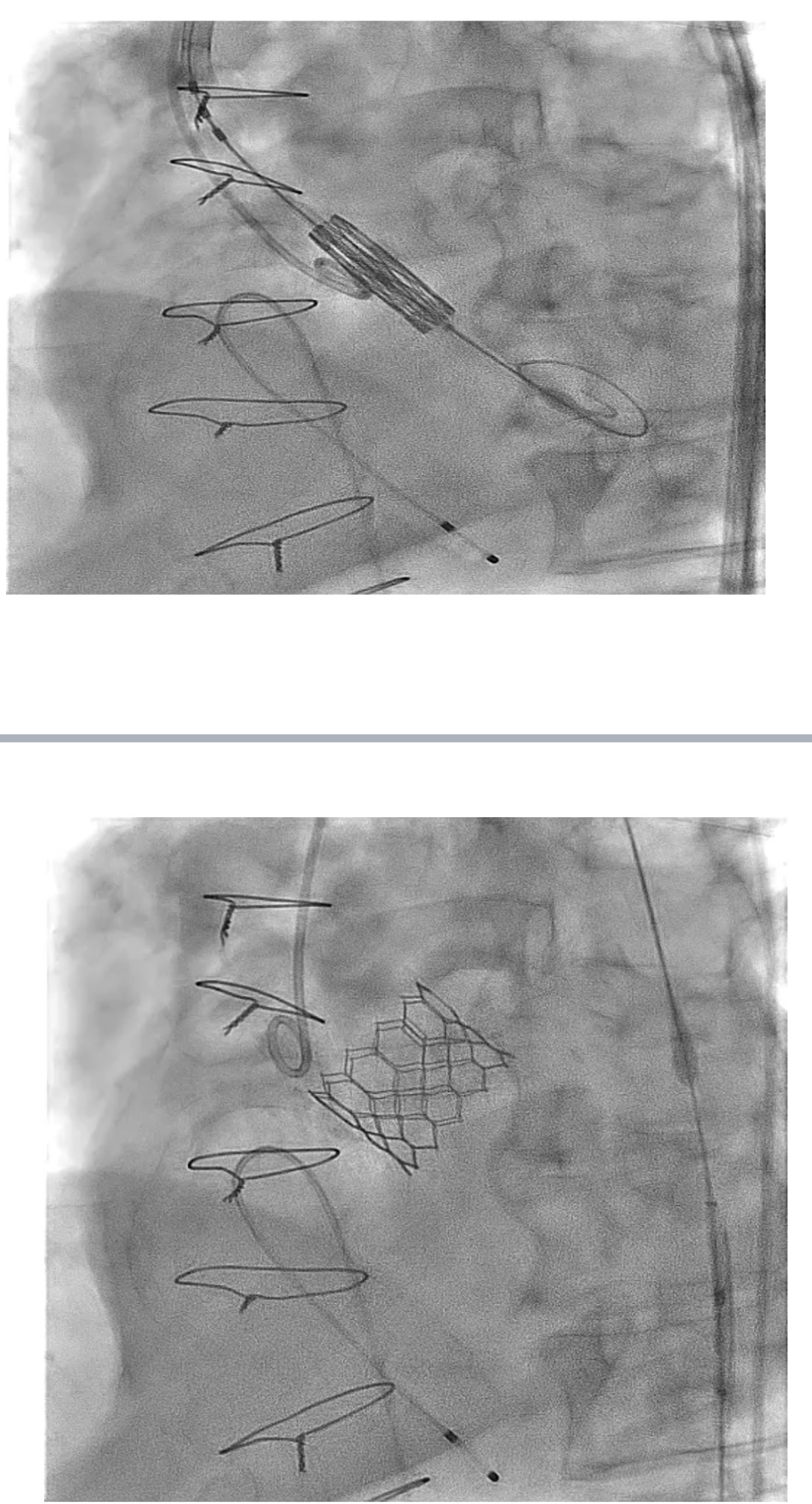
Following informed consent, the TAVI procedure was performed in the hybrid catheterization lab via a standard transfemoral approach. Femoral sheaths were inserted under fluoroscopic and ultrasound guidance, and the aortic valve was crossed with a Confida wire placed in the left ventricle. A pigtail catheter was positioned in the non-coronary cusp, and a baseline aortogram was performed using Gadobutrol administered via an automated injector. A 29 mm Sapien 3 Ultra valve (Edwards Lifesciences, Irvine, California) was deployed after confirming its position with a Gadobutrol aortogram. The image quality was comparable to iodinated contrast media (ICM), and the procedure was successfully completed without any adverse reactions to Gadobutrol.
Post-TAVI aortography confirmed proper valve positioning with only a trivial paravalvular leak (PVL). The access sites were successfully closed using ProGlide and Angio-Seal devices. A total of 50 cc of Gadobutrol was used. Pre-discharge transthoracic echocardiography (TTE) confirmed good transcatheter heart valve (THV) positioning, no PVL, and a peak gradient of 6 mmHg. Renal function tests performed one week post-procedure showed no deterioration, with a creatinine level of 156 µmol/L.



Case Summary
Gadolinium can be safely used to guide TAVI procedure in patients with contraindications to the use of ICM. It provides acceptable image quality especially when used with a power injector, which improve the images quality and reduces the amount of gadolinium administered.


