TCTAP A-091
Pharmacotherapy (Heart Failure)
The Role of Cardioprotective Medications in Preventing Chemotherapy-Induced Cardiotoxicity in Breast Cancer Patients: A Bayesian Network Meta-Analysis of Randomized Controlled Trials
Ali Al-Shammari1, Ahmed Ibrahim2, M Rafiqul Islam3, Pakeezah Tabasum4, Rubab Zahra5, Naiela E. Almansouri6, Hadeel Aboueisha7, Areeba Ahsan8, Hiba Hamdar9, Ahmed Sermed Al Sakini1, Uzair Iqbal5, Rida Bashir10, Sura N. Alrubaye11, Mohammed Marsool12, Sara Shihab13
University of Baghdad, Iraq1, Alexandria University, Egypt2, Shaheed Suhrawardy Medical College and Hospital, Bangladesh3, People's University of Medical and Health Sciences for Women, Pakistan4, Allama Iqbal Medical College, Pakistan5, University of Tripoli, Libya6, Suez Canal Universiy, Egypt7, Foundation University, Pakistan8, Medical Learning Skills Academy, Lebanon9, Shalamar Hospital, Pakistan10, University of Babylon, Iraq11, University of Baghdad, Iraq12, Mayo Clinic, USA13
Background
Chemotherapy-induced cardiotoxicity remains a major concern for breast cancer patients undergoing chemotherapy, often leading to declines in the left ventricular ejection fraction (LVEF) and subsequent heart failure. Prior research indicates that cardioprotective medications may alleviate these detrimental cardiac effects. We aim to evaluate the comparative effectiveness and safety of cardioprotective medications in preserving LVEF in breast cancer patients on chemotherapy.
Methods
Following PRISMA guidelines, we searched several electronic databases, including Cochrane, PubMed, WOS, and SCOPUS up to August 4, 2024, to find randomized controlled trials (RCTs) that assessed the effectiveness and safety of cardioprotective drugs including beta-blockers, angiotensin-converting enzyme (ACE) inhibitors, angiotensin receptor blockers (ARBs), and statins in breast cancer patients undergoing chemotherapy. Bayesian network meta-analysis was performed using the BUGSnet package in Rstudio version 4.4.2, applying a random-effects model, and mean differences (MD) with 95% credible intervals (CrI) were calculated. Interventions were ranked based on the surface under the cumulative ranking curve (SUCRA) values and higher SUCRA values indicate greater effectiveness.
Results
The analysis included 18 RCTs, comprising 2,223 breast cancer patients undergoing chemotherapy who received various cardioprotective medications. Study findings indicate that Placebo (SUCRA: 86.46) served as the reference, with MD of 0.0 (95% CrI: 0,0). Among the active interventions, Spironolactone demonstrated the largest effect size, with MD of 12.79 (95% CrI: 5.99, 19.57) and a low SUCRA ranking (2.16), indicating its effectiveness despite a lower ranking. Similarly, Nebivolol (SUCRA = 18.25) showed significant effectiveness, with an MD of 7.29 (95% CrI: 0.34, 14.23). Other treatments included Lisinopril+Bisoprolol (SUCRA = 30.6), which had MD of 5.25 (95% CrI: -1.57, 12.06), and Rosuvastatin (SUCRA = 42.67), which demonstrated MD of 3.64 (95% CrI: -2.48, 9.76). While both showed promising effect sizes, their CrIs included zero, indicating moderate uncertainty. Ramipril+Bisoprolol (SUCRA = 46.63, MD = 3.14, 95% CrI: -2.40, 8.67) also showed moderate efficacy, though its CrI included zero. Beta-blockers like Bisoprolol (SUCRA = 46.39) and Candesartan (SUCRA = 48.3) exhibited modest effectiveness, with MDs of 3.04 (95% CrI: -1.18, 7.28) and 2.80 (95% CrI: -0.45, 5.99), respectively. Candesartan+Metoprolol (SUCRA = 51.32, MD = 2.66, 95% CrI: -2.69, 7.95) showed similar effects, but all three interventions are statistically insignificant. Among ACE inhibitors, Ramipril (SUCRA = 55.09) demonstrated MD of 2.38 (95% CrI: -3.12, 7.87), while Perindopril (SUCRA = 64.32) and Lisinopril (SUCRA = 61.62) had MDs of 1.52 (95% CrI: -3.99, 7.06) and 1.76 (95% CrI: -3.11, 6.64), respectively, with minimal clinical impact. Atorvastatin (SUCRA = 52.66, MD = 2.60, 95% CrI: -3.41, 8.61) and Carvedilol (SUCRA = 71.05, MD = 1.09, 95% CrI: -0.82, 3.03) showed negligible improvements. Metoprolol (SUCRA = 72.42) had the lowest MD (0.76, 95% CrI: -4.55, 6.01) and minimal effect on LVEF (Figures 1, 2, and 3). The drugs were well-tolerated with no significant adverse effects.
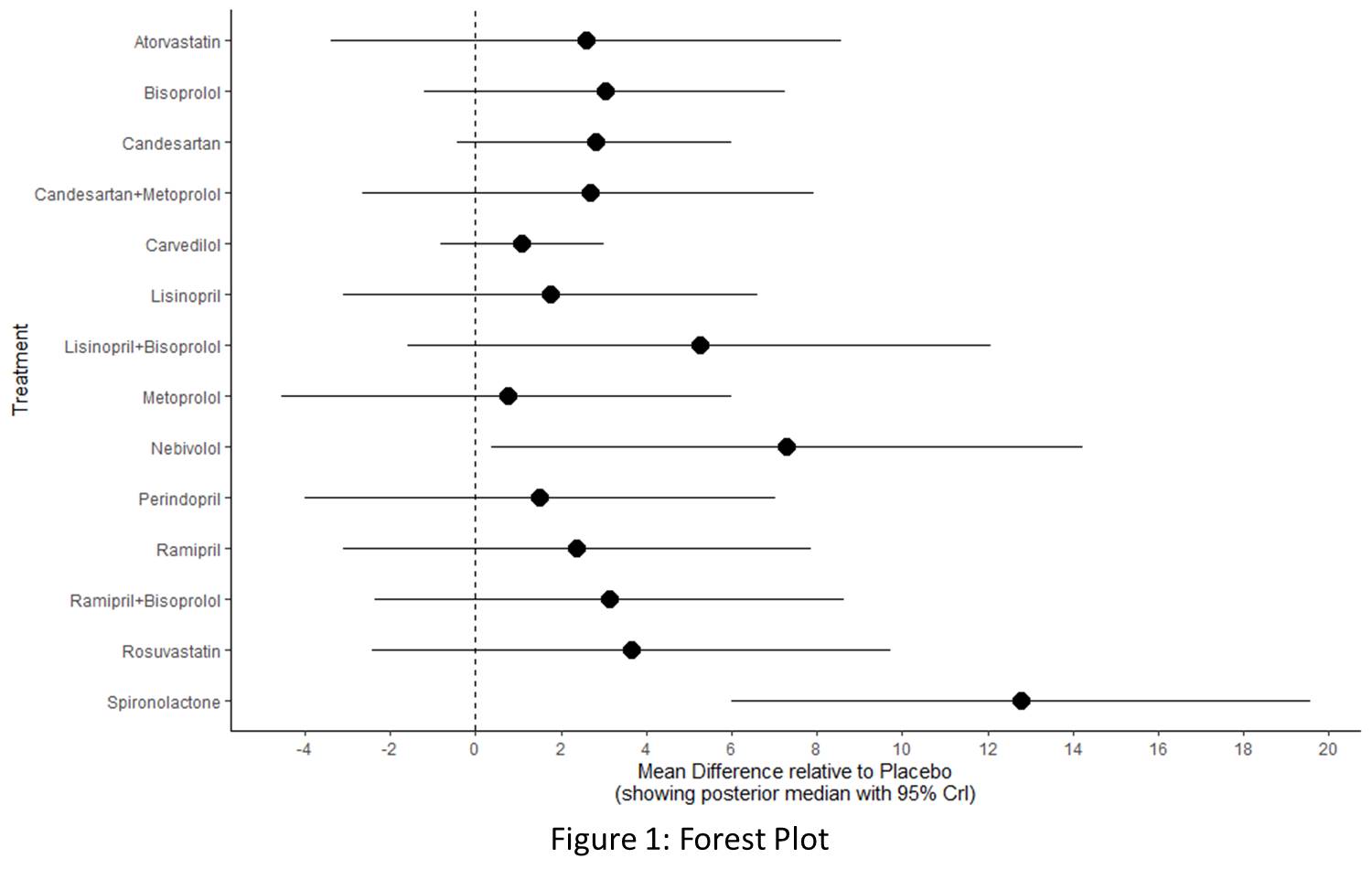
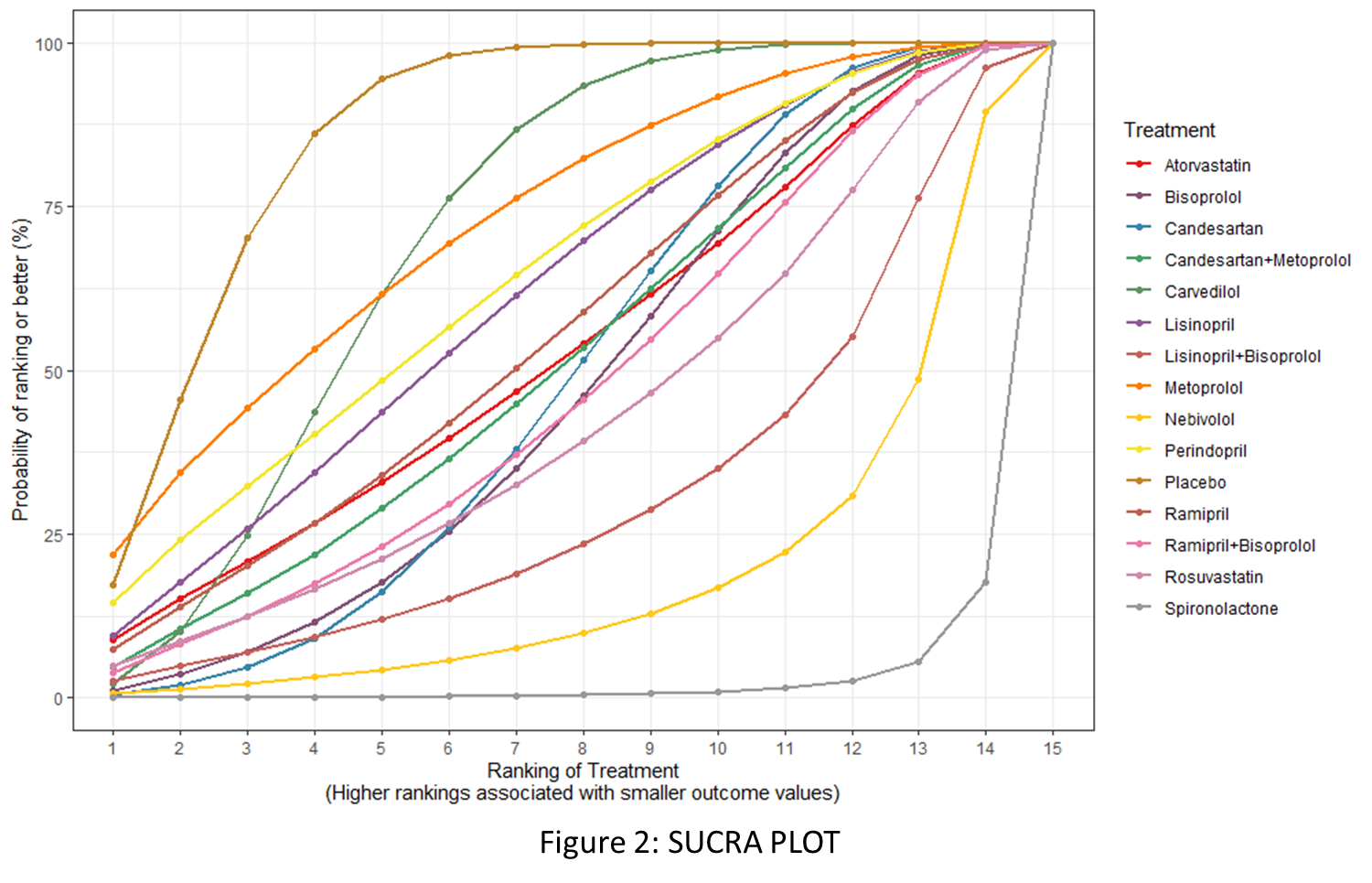
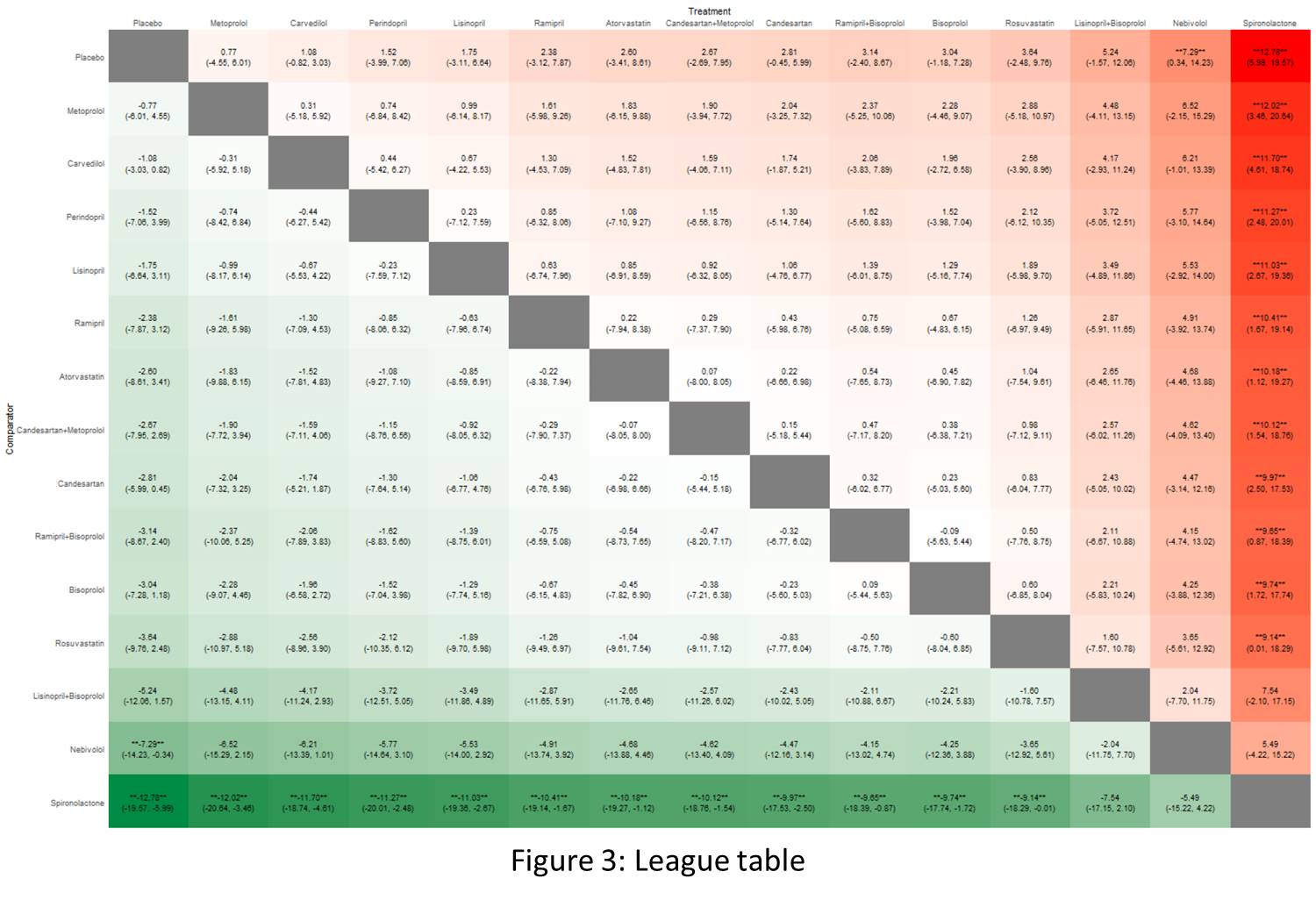
Conclusion
These findings suggest that Spironolactone and Nebivolol are promising candidates for preserving LVEF in breast cancer patients undergoing chemotherapy, while Metoprolol showed minimal benefit. However, further studies with larger sample sizes and longer follow-up are essential to validate these results and assess long-term clinical outcomes.





