Lots of interesting abstracts and cases were submitted for TCTAP 2025. Below are the accepted ones after a thorough review by our official reviewers. Don’t miss the opportunity to expand your knowledge and interact with authors as well as virtual participants by sharing your opinion in the comment section!
TCTAP A-031
The Use of Colchicine After Coronary Artery Stenting: One-Year Follow-Up Results
By Sherzod Ahmedov, Ilhom Jurayev, Muzaffar Juraliyev, Ahrorov Javoxir, Shermuhammad Tursunov, Baxromov Behruz
Presenter
Sherzod Ahmedov
Authors
Sherzod Ahmedov1, Ilhom Jurayev2, Muzaffar Juraliyev1, Ahrorov Javoxir2, Shermuhammad Tursunov2, Baxromov Behruz2
Affiliation
Ezgu Niyat, Uzbekistan1, Carmen Plus Hospital, Uzbekistan2
View Study Report
TCTAP A-031
In-Stent Restenosis
The Use of Colchicine After Coronary Artery Stenting: One-Year Follow-Up Results
Sherzod Ahmedov1, Ilhom Jurayev2, Muzaffar Juraliyev1, Ahrorov Javoxir2, Shermuhammad Tursunov2, Baxromov Behruz2
Ezgu Niyat, Uzbekistan1, Carmen Plus Hospital, Uzbekistan2
Background
Restenosis following percutaneous coronary intervention (PCI) remains a significant challenge in cardiovascular care, often leading to recurrent angina, myocardial infarction, and the need for repeat revascularization. Despite advances in stent technology, including drug-eluting stents (DES), restenosis still occurs in a subset of patients, particularly those with diabetes, vascular calcification, and other high-risk factors.Inflammation plays a key role in the pathogenesis of restenosis. Following PCI, endothelial injury and inflammatory responses contribute to neointimal hyperplasia, leading to vessel re-narrowing. Targeting inflammation has emerged as a potential strategy to reduce restenosis and improve long-term outcomes in PCI patients.Colchicine, an anti-inflammatory agent traditionally used for gout, has gained attention for its potential to modulate the inflammatory pathways involved in atherosclerosis and restenosis. Studies have suggested that colchicine can reduce the incidence of cardiovascular events by inhibiting NLRP3 inflammasome activation, which is implicated in inflammation and plaque rupture. Recent trials, such as theCOLCOT (Colchicine Cardiovascular Outcomes Trial), have shown promising results, indicating that colchicine may help reduce major adverse cardiovascular events (MACE) and improve outcomes in high-risk patients.The ESC and ACC guidelines advocate for strategies that reduce inflammation, particularly in patients with high cardiovascular risk. While colchicine’s role in reducing MACE is recognized, its specific effects on restenosis after PCI remain under investigation. This study aims to evaluate the efficacy of colchicine in reducing restenosis following PCI and explore its potential as an adjunctive therapy in reducing the long-term complications of coronary artery disease.
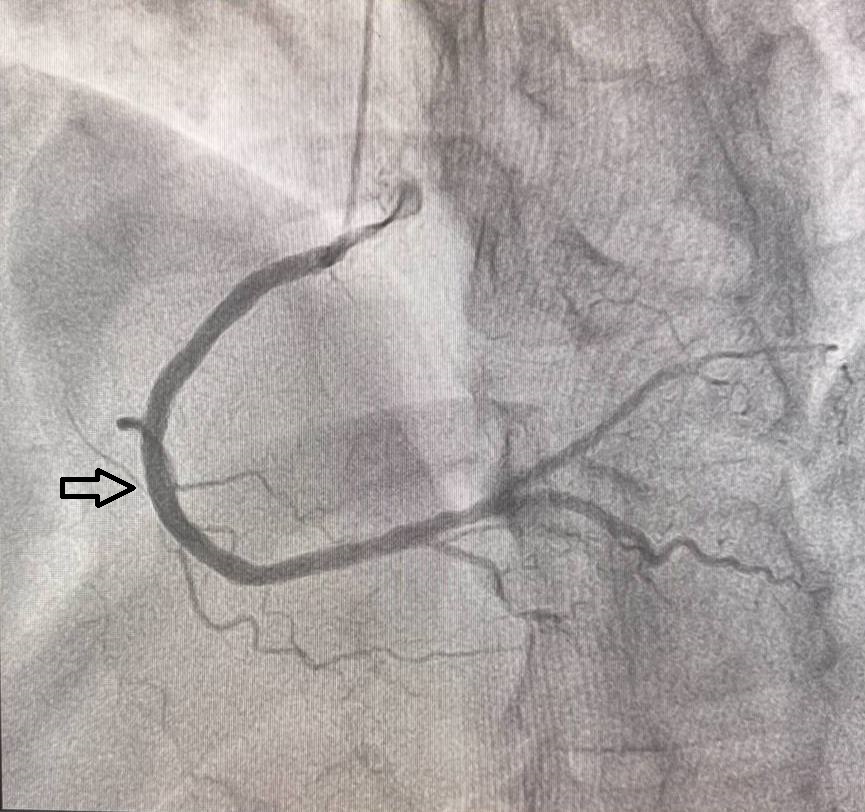
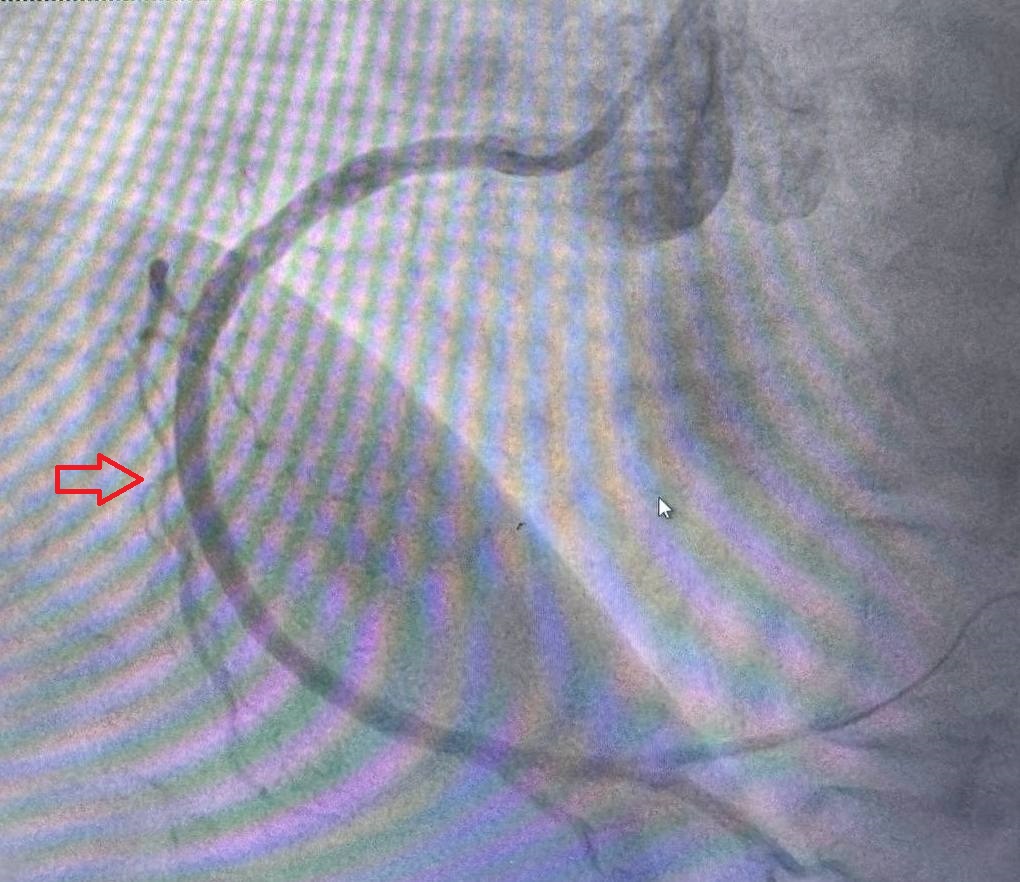


Methods
This study was a prospective, randomized, controlled trial conducted at "Ezgu niyat" and "Carmen +" clinics between 2021 - 2024. A total of 120 patients undergoing elective PCI for coronary artery disease were enrolled. Patients were randomly assigned to receive either colchicine (0.5 mg daily) or a placebo for 12 months post-PCI. The inclusion criteria were patients aged 18-75, with stable coronary artery disease, who underwent successful PCI with drug-eluting stent implantation. Exclusion criteria included hypersensitivity to colchicine, active infections, severe renal or hepatic impairment, and recent history of stroke or myocardial infarction.
Study Design
- Group 1 (Colchicine group): 60 patients received colchicine 0.5 mg daily for 12 months.
- Group 2 (Placebo group): 60 patients received a placebo with the same regimen for 12 months.
Primary Outcome
The primary endpoint was the incidence of restenosis at 12 months, assessed by coronary angiography. Restenosis was defined as ≥50% diameter stenosis in any coronary artery segment at the site of the stent. Angiography was performed in two projections both before implantation and after 12 months, using the same angle to ensure consistency.Secondary Outcomes
Secondary outcomes included major adverse cardiovascular events (MACE), such as non-fatal myocardial infarction, repeat revascularization, and death. Inflammatory markers (C-reactive protein, IL-6, and TNF-α) were also measured at baseline and 12 months to evaluate the effect of colchicine on systemic inflammation.Statistical Analysis
The data were analyzed using descriptive statistics, chi-square tests for categorical variables, and t-tests for continuous variables. The p-value for statistical significance was set at <0.05. Kaplan-Meier survival curves were used to estimate the time to restenosis, and Cox proportional hazards models were used to identify independent predictors of restenosis.Results
One-Year Follow-UpCoronary Angiography Results
ColchicineGroup
Of the 60 patients who received colchicine (0.5mg daily), restenosis was observed in 4 patients (6.7%).
PlaceboGroup
In the placebo group, restenosis was observedin 11 out of 60 patients (18.3%).
Key Statistical Findings
The p-value for restenosis betweenthe colchicine and placebo groups was 0.02, indicating a statisticallysignificant reduction in restenosis in the colchicine group.
Observations
Anti-Inflammatory Effects: The reduction inrestenosis rates in the colchicine group is attributed to the drug's stronganti-inflammatory properties.
Conclusion
The use of colchicine (0.5 mg daily) significantly reduced the rate of restenosis compared to the placebo group, with a statistically significant p-value of 0.02. This result is consistent with recent meta-analyses showing that colchicine’s anti-inflammatory properties can help reduce vascular inflammation, a key factor in restenosis and other cardiovascular events.The ESC (European Society of Cardiology) and ACC (American College of Cardiology) guidelines recommend anti-inflammatory therapies for improving outcomes in patients with atherosclerotic cardiovascular disease (ASCVD). Colchicine’s role in reducing inflammation following PCI aligns with these recommendations, particularly for high-risk populations, such as patients with diabetes and vascular calcification.In the colchicine group, the restenosis rate was significantly lower across all vascular territories, with no restenosis in LAD and LCX, and a minimal rate in RCA. These results are in line with the COLCOT (Colchicine Cardiovascular Outcomes Trial) and other large studies, which demonstrated that colchicine reduces major adverse cardiovascular events (MACE) and improves long-term outcomes.Patients with vascular calcification, traditionally at higher risk for restenosis, experienced considerable benefit from colchicine therapy. This finding supports the ESC Guidelines for the Management of Chronic Coronary Syndromes, which emphasize personalized treatment strategies targeting inflammation in high-risk patients. In contrast, the placebo group showed a higher rate of restenosis, especially in vessels with significant calcification and among diabetic patients, consistent with ACC/AHA guidelines highlighting increased restenosis risk in these subgroups.These findings suggest that colchicine could be a valuable adjunctive therapy in PCI to reduce restenosis, particularly for patients at high risk of adverse outcomes. Future studies with larger cohorts are needed to confirm these results and optimize patient selection for colchicine therapy.


