Lots of interesting abstracts and cases were submitted for TCTAP 2025. Below are the accepted ones after a thorough review by our official reviewers. Don’t miss the opportunity to expand your knowledge and interact with authors as well as virtual participants by sharing your opinion in the comment section!
TCTAP A-048
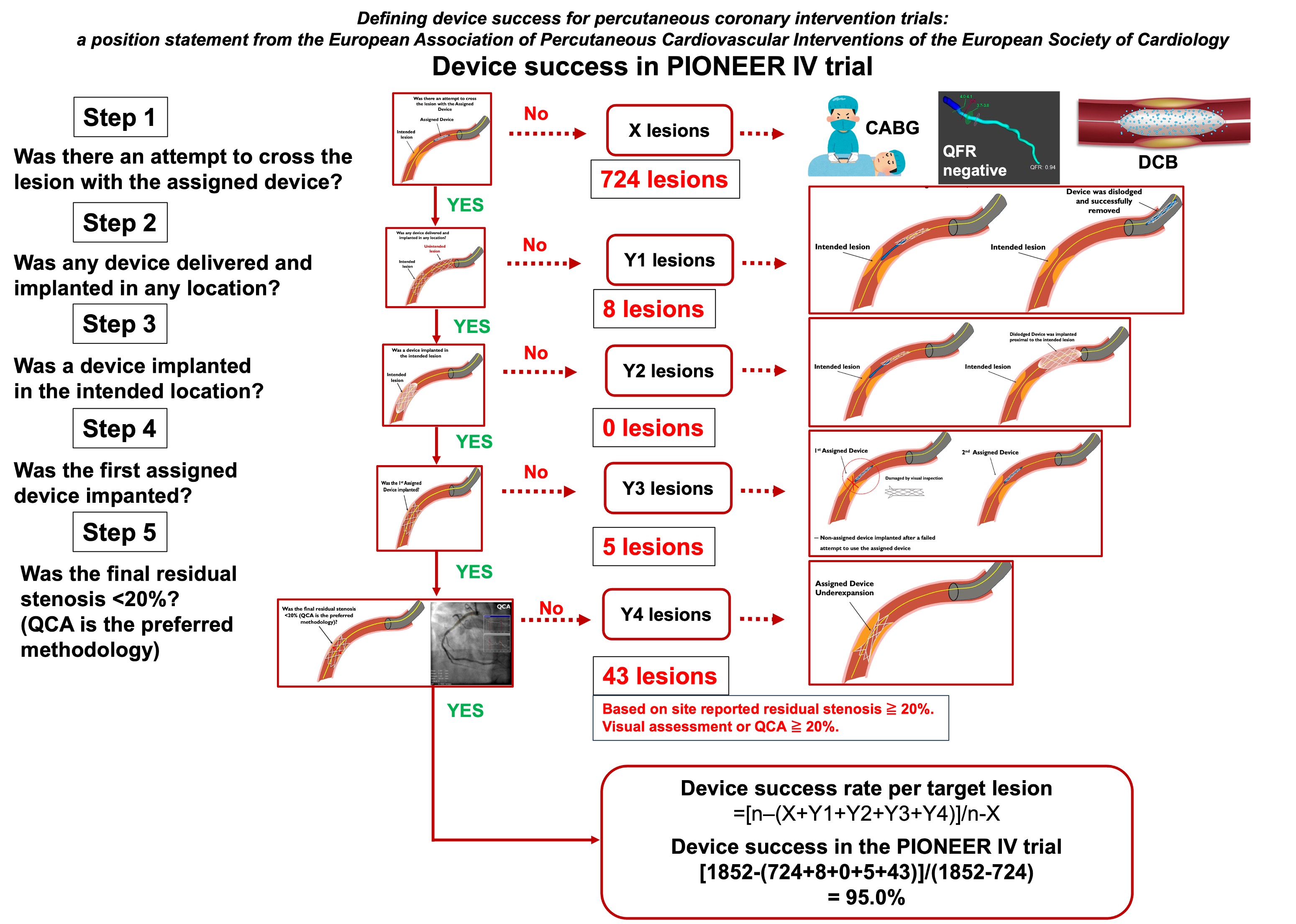
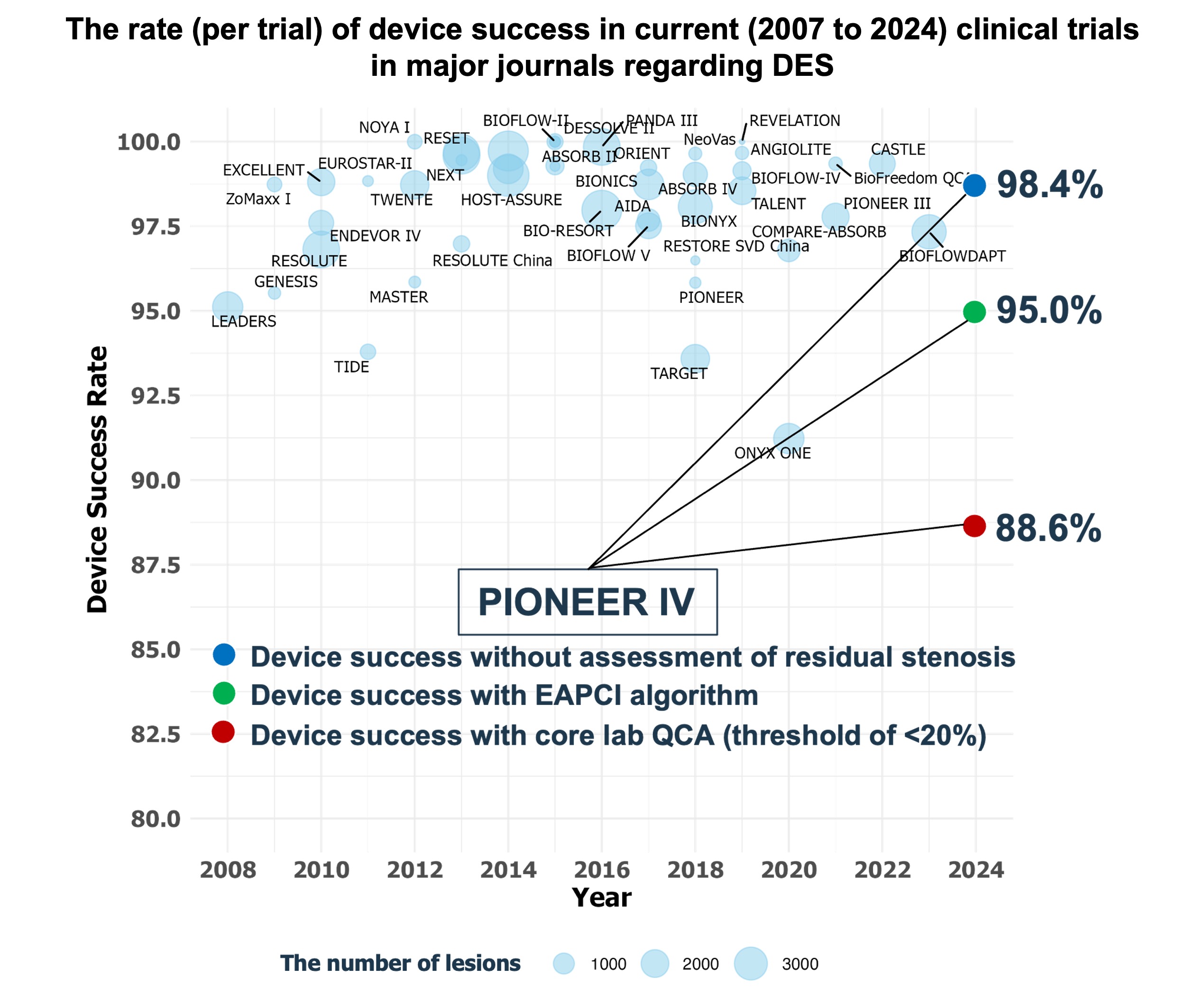
Device Success Algorithm of the European Association of Percutaneous Cardiovascular interventions (EAPCI) Prospectively Applied to the Pioneer IV Trial
By Asahi Oshima, Tsung-Ying Tsai, Joanna Wykrzykowska, Faisal Sharif, Liesbeth Rosseel, Mohammad Alkhalil, Edouard Benit, Nick Curzen, Vincent Flore, Kenneth De Wilder, Valeria Paradies, Giovanni Amoroso, Sjoerd Hofma, Adel Aminian, Clemens von Birgelen, Manel Sabate, Victor Jimenez Diaz, Ignacio Amat Santos, Jeroen Sonck, Anthony Mathur, Lionel Mangin, Mathieu Pankert, Gilles Barone-Rochette, Julien Lemoine, Michael Angioi, Raymundo Ocaranza, Julien Adjedj, Albert Chinhenzva, Shiuan-Hao Hu, Emelyne Sevestre, Pruthvi C. Revaiah, Akihiro Tobe, Patrick Serruys, Yoshinobu Onuma
Presenter
Authors
Affiliation
Device Success Algorithm of the European Association of Percutaneous Cardiovascular interventions (EAPCI) Prospectively Applied to the Pioneer IV Trial
Asahi Oshima1, Tsung-Ying Tsai1, Joanna Wykrzykowska2, Faisal Sharif1, Liesbeth Rosseel3, Mohammad Alkhalil4, Edouard Benit5, Nick Curzen6, Vincent Flore7, Kenneth De Wilder8, Valeria Paradies9, Giovanni Amoroso10, Sjoerd Hofma11, Adel Aminian12, Clemens von Birgelen13, Manel Sabate14, Victor Jimenez Diaz15, Ignacio Amat Santos16, Jeroen Sonck17, Anthony Mathur18, Lionel Mangin19, Mathieu Pankert20, Gilles Barone-Rochette21, Julien Lemoine22, Michael Angioi22, Raymundo Ocaranza23, Julien Adjedj24, Albert Chinhenzva1, Shiuan-Hao Hu1, Emelyne Sevestre1, Pruthvi C. Revaiah1, Akihiro Tobe1, Patrick Serruys1, Yoshinobu Onuma1
University of Galway, Ireland1, University Medical Center Groningen, Netherlands2, Algemeen Stedelijk Ziekenhuis, Belgium3, Freeman Hospital, United Kingdom4, Jessa Hospital, Belgium5, University Hospital Southampton NHS Trust, United Kingdom6, Algemeen Ziekenhuis Maria Middelares, Belgium7, Imelda Hospital, Belgium8, Maasstad Hospital, Netherlands9, OLVG Amsterdam, Netherlands10, Medisch Centrum Leeuwarden, Netherlands11, Centre Hospitalier Universitaire de Charleroi, Belgium12, Medisch Spectrum Twente, Netherlands13, University of Barcelona, Spain14, Hospital Alvaro Cunqueiro, Spain15, Hospital Clinico Universitario de Valladolid, Spain16, OLV Clinic, Belgium17, Queen Mary University of London, United Kingdom18, Hospital Annecy-Genevois, France19, Centre Hospitalier Avignon, France20, CHU Grenoble Alpes, France21, Clinique Louis Pasteur, France22, Hospital Lucus Augusti, Spain23, Arnault Tzanck Institute, France24
Background
Methods
Results