Lots of interesting abstracts and cases were submitted for TCTAP 2025. Below are the accepted ones after a thorough review by our official reviewers. Don’t miss the opportunity to expand your knowledge and interact with authors as well as virtual participants by sharing your opinion in the comment section!
TCTAP A-069
Long-Term Impact of Early Subclinical Leaflet Thrombosis After Transcatheter Aortic Valve Implantation
By Juri Iwata, Kentaro Hayashida
Presenter
Juri Iwata
Authors
Juri Iwata1, Kentaro Hayashida1
Affiliation
Keio University Hospital, Japan1
View Study Report
TCTAP A-069
Computed Tomography
Long-Term Impact of Early Subclinical Leaflet Thrombosis After Transcatheter Aortic Valve Implantation
Juri Iwata1, Kentaro Hayashida1
Keio University Hospital, Japan1
Background
The mid-term outcomes of transcatheter aorticvalve implantation (TAVI) have been well established, and its long-term safety and efficacy remain unclear, given the expandingindication of TAVI inyounger and lower-risk patients. The utmost concerns in young or low-riskpatients are valve durability and long-term clinical outcomes, as thesepatients tend to require longer valve durability and reintervention.
Methods
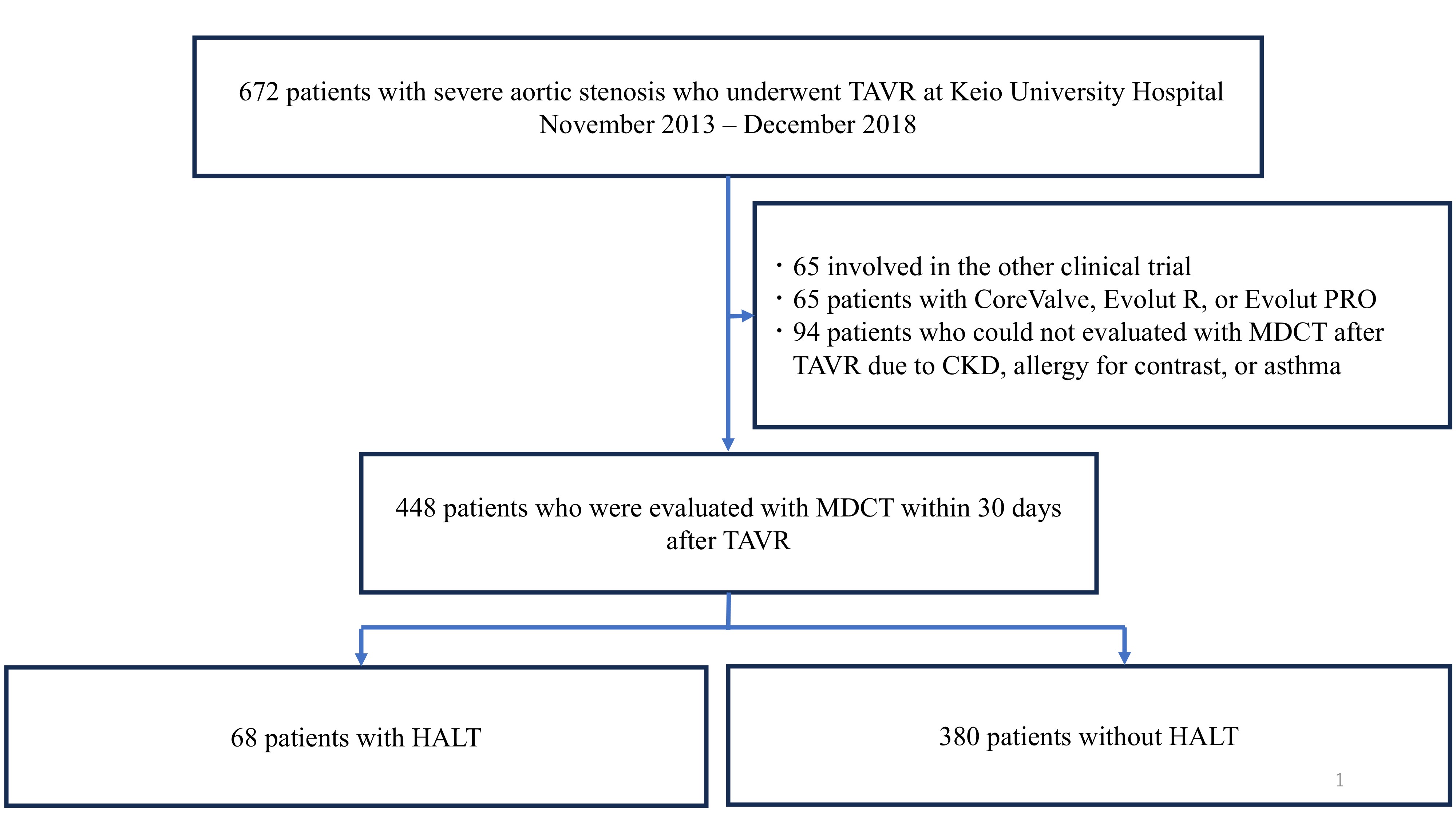
Of the 672 consecutive patients who underwent TAVI between2013 and 2018 at Keio University Hospital, we included 448 who were treatedwith either SAPIEN XT or SAPIEN 3 and underwent MDCT analysis within 30 daysafter TAVI. We excluded patients who did not undergo MDCT after TAVI due tosevere chronic kidney disease, allergy to contrast, or asthma, participated inother clinical trials, or received CoreValve, Evolut R, or Evolut PRO. Regularfollow-ups were scheduled at the time of one month and annually thereafter. Themain endpoint of this study was all-cause mortality beyond six years afterTAVI. Other clinical endpoints, including cardiovascular death, stroke,rehospitalization for heart failure, hemodynamic performance, and SVD, werealso evaluated using echocardiography.

Results
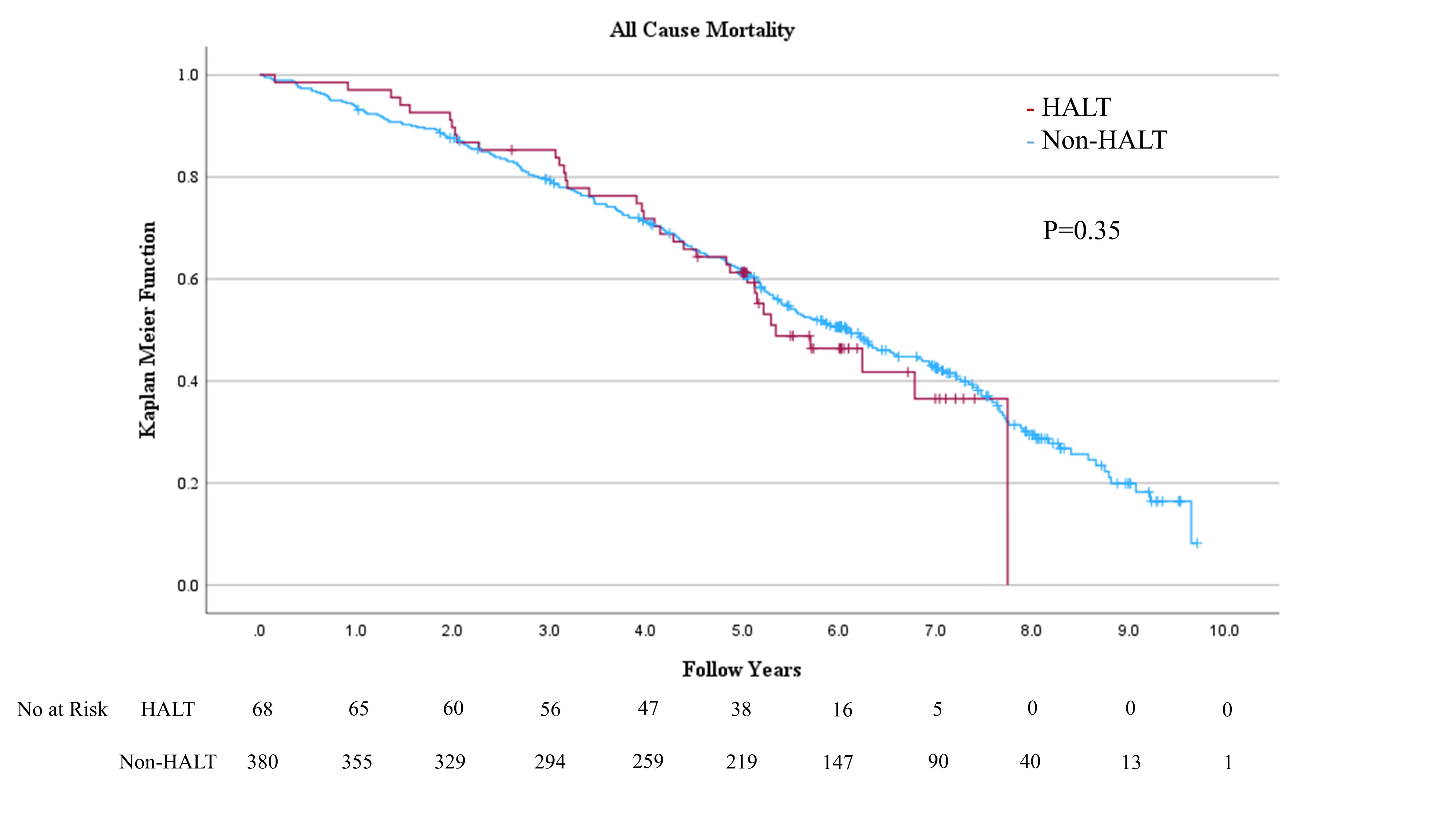
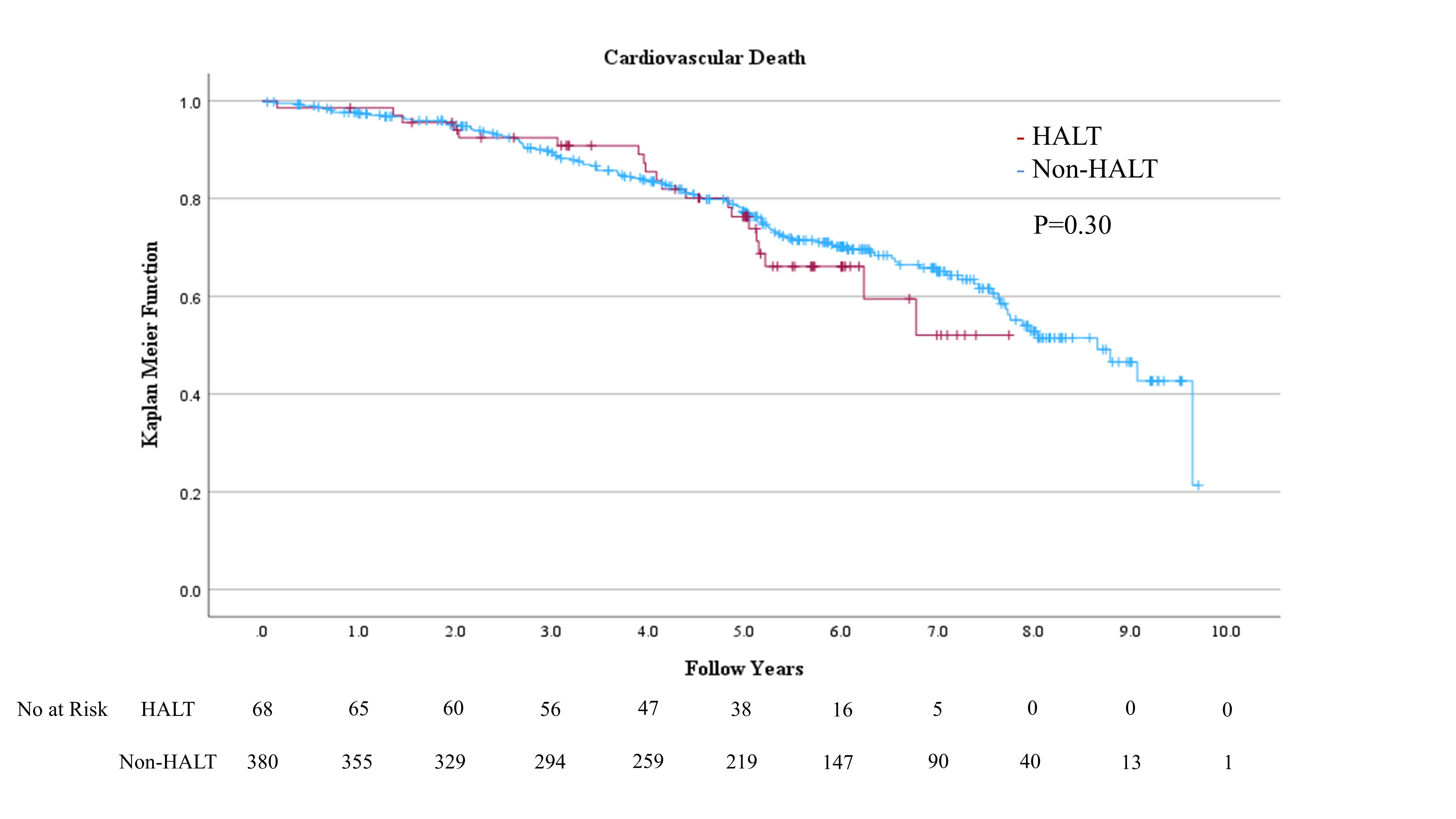
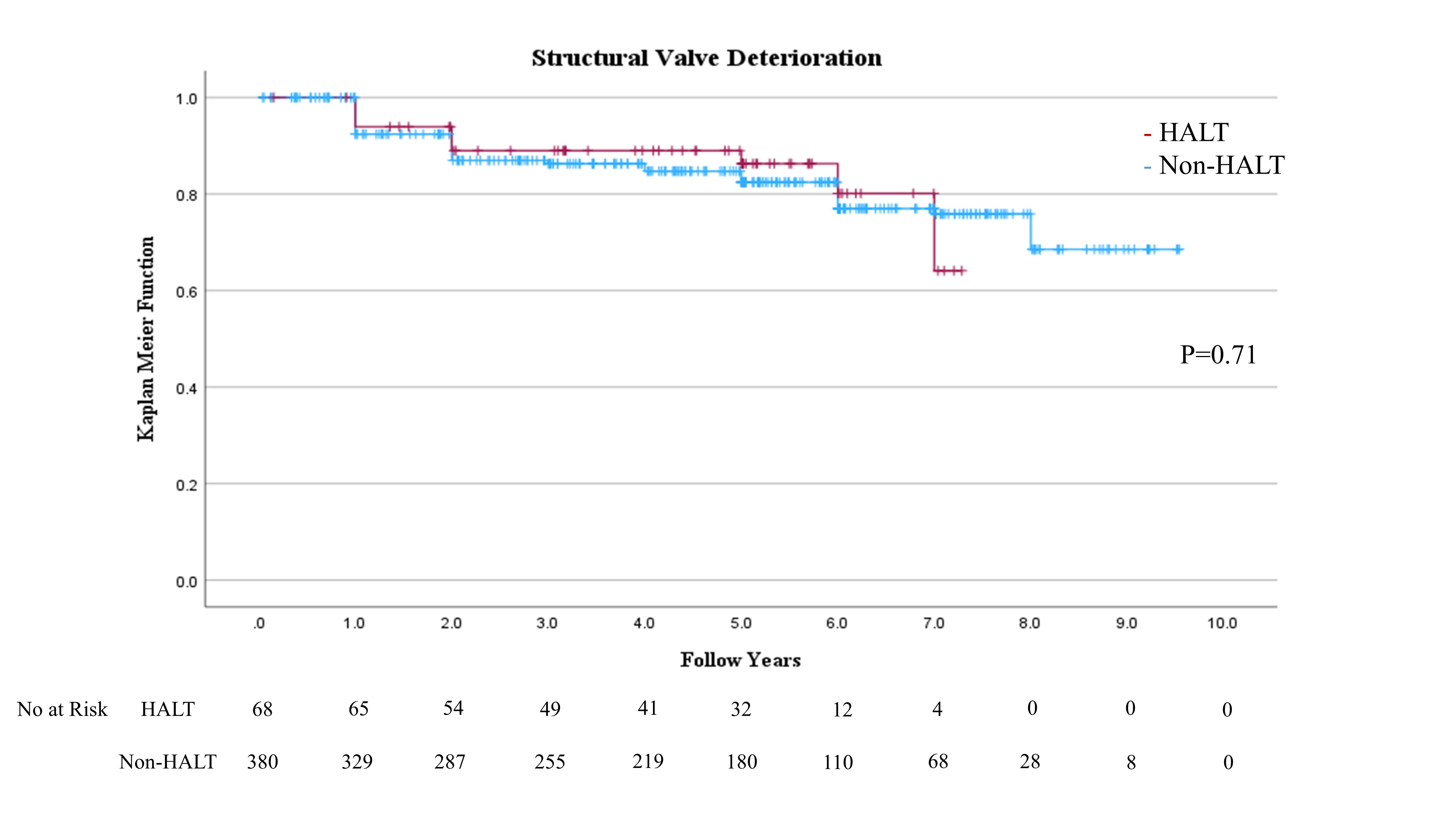
HALT was detected in 68 (15.2%) of 448 eligible patients within 30 daysafter TAVI. No significant difference in hemodynamic performance was observed by echocardiography within 30 days after TAVI between the HALT and the non-HALT groups ineach valve type (SAPIEN XT: effective orifice area: 1.62±0.66 cm2 vs. 1.72±0.43 cm2; P=0.26, SAPIEN 3: 1.42±0.35 cm2 vs. 1.45±0.34 cm2; P=0.63). No significant differences were observed inthe incidences of procedural complications, including coronary obstruction,disabling stroke, major or life-threatening bleeding, major vascularcomplications, or new permanent pacemaker implantation, between the groups. Noadditional antithrombotic therapy was administered at the time of discharge forpatients with HALT due to the absence of dyspnea. Over six years, all-cause mortality (52.9% vs.60.0%; hazard ratio (HR): 1.19; 95% confidence interval (CI): 0.83-1.70; P=0.3), stroke incidence (5.9% vs. 7.1%;HR: 1.06; 95% CI: 0.08-13.7; P=0.97),heart failure rehospitalization (10.3% vs. 15.0%; HR: 2.3; 95% CI: 0.89-5.99; P=0.09), and SVD (14.7% vs. 17.9%;HR: 0.89; 95% CI: 0.45-1.73; P=0.73)were comparable between the HALT and the non-HALT groups during themedian follow-up of 1,872 (interquartile range; 1,203-2,468) days. During the annual follow-up by echocardiography, nosignificant difference was observed in effective orifice area and mean pressuregradient between the two groups in each year.



Conclusion
To the best of our knowledge, our study is the first to evaluatelong-term outcomes beyond six years for HALT in comparison with non-HALT. This study showed HALT at 30days in approximately 15.2% of the TAVI procedures. The salient findings fromthe present analysis comparing patients with and without HALT are summarized asfollows: (1)SAPIEN 3 was administeredmore frequently to the HALT group than to the non-HALT group. (2) No significant differences were observed in hemodynamic performance by echocardiography within 30 daysafter TAVI between the HALT and the non-HALT groups in each valve type. (3) Moreover, no significant differences were observed inlong-term clinical outcomes, including all-cause mortality, stroke, and heart failure rehospitalization during more than six yearsof follow-up. (4) More than six years after TAVI,the development of SVD was comparable between the HALT and the non-HALT groups. Thisstudy indicated that HALT within 30 days after TAVI was not associated with long-term mortality, stroke, heart failurerehospitalization risks, or hemodynamic performance for more than six years.These findings suggest that THV has excellent long-term durability, even whenconsidering HALT.


