
Lawrence A. Garcia
St. Francis Hospital
Chronic limb-threatening ischemia (CLTI), a condition of arterial insufficiency of the lower limb with ischemic foot pain at rest, a nonhealing ischemic ulcer, or gangrene, is the most severe form of peripheral arterial disease, and its optimal initial treatment—whether it°Įs surgical or endovascular revascularization—remains a topic of debate.
At TCTAP 2024, Lawrence A. Garcia, MD (St. Francis Hospital and Heart Center, Roslyn, NY, USA) provided insights into this issue by analyzing details from two landmark trials, BEST-CLI and BASIL-2 (Figure 1). Published a year ago at around the same time, these studies presented contradictory conclusions about patients with CLTI, thus causing considerable debate.
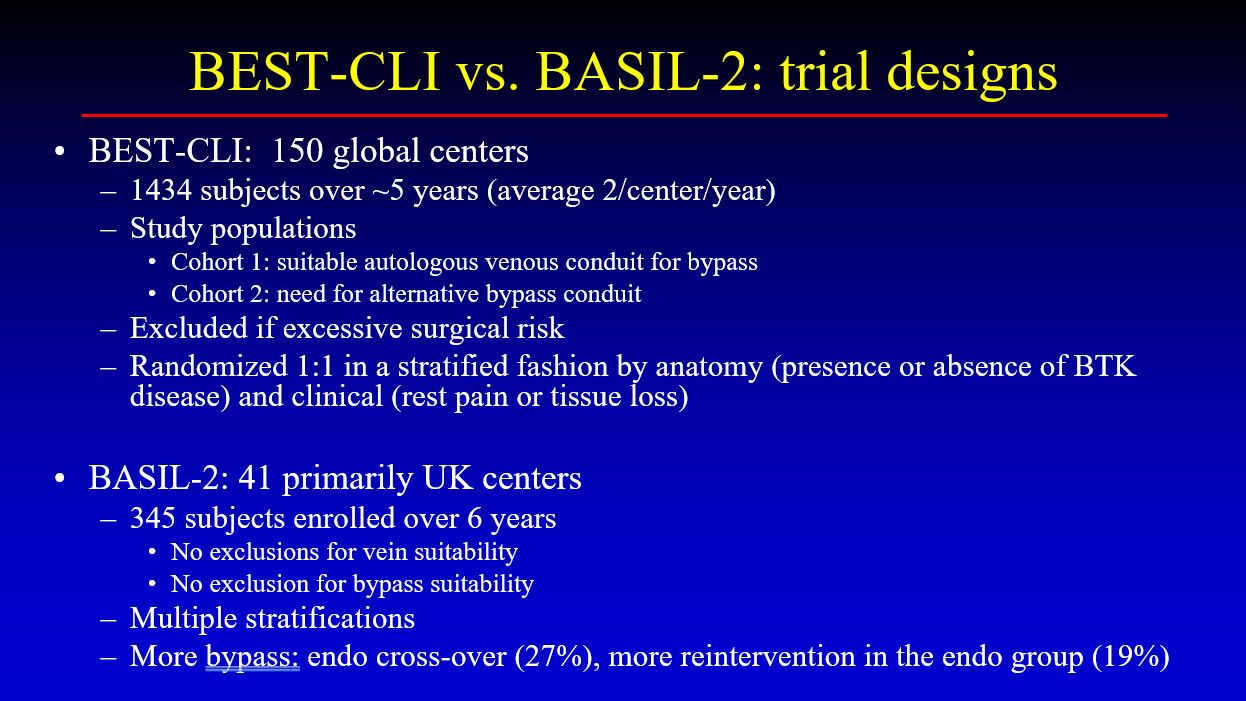
The BEST-CLI study was a randomized superiority trial that enrolled 1,847 patients across 150 sites in the USA, Canada, Finland, Italy, and New Zealand. It consisted of two cohorts: cohort 1 included 1,434 patients with suitable venous conduits for bypass (718 undergoing surgical revascularization and 716 receiving endovascular treatment), and cohort 2 included 396 patients requiring alternative conduits (197 undergoing surgical revascularization and 199 receiving endovascular treatment). In cohort 1, the median time to the index procedure was 4 days (interquartile range [IQR], 1-11 days) for the surgical group and 1 day (IQR, 0-7 days) for the endovascular group. In the surgical group, 85% of the procedures utilized a single segment of the great saphenous vein, achieving a technical success rate of 98%, compared to 85% in the endovascular group.
In cohort 2, the median time to the index procedure was 4 days (IQR, 1-13 days) for the surgical group and 1 day (IQR, 0-7 days) for the endovascular group, with technical success rates of 100% and 80.6%, respectively. During a median follow-up of 2.7 years in cohort 1, 42.6% of the surgical group and 57.4% of the endovascular group experienced the primary outcome of major adverse limb events (MALE) or death (hazard ratio [HR], 0.68; 95% confidence interval [CI], 0.59-0.79; p< .001). The surgical group also had fewer major reinterventions and above-ankle amputations than the endovascular group (9.2% and 10.4% vs. 23.5% and 14.9%, respectively).
In the BASIL-2 study, patients with CLTI requiring infrapopliteal revascularization who received the best endovascular treatment had better amputation-free survival (AFS) than those who received surgical vein bypass. Conducted across 41 hospital-based vascular surgery units in the United Kingdom, Sweden, and Denmark, the study had no exclusions for vein or bypass suitability and involved 345 patients. Major amputation or death occurred in 63% of patients (108 out of 172) in the vein bypass group versus 53% (92 out of 173) in the best endovascular treatment group (adjusted HR, 1.35; 95% CI, 1.02-1.80; p = .037). The median AFS was 3.3 years (IQR, 2.1-4.3 years) in the vein bypass group and 4.4 years (IQR, 3.4-5.9 years) in the best endovascular treatment group. Death from any cause occurred in 53% of the vein bypass group and 45% of the best endovascular treatment group (adjusted HR for overall survival, 1.37; 95% CI, 1.00-1.87). Major amputation occurred in 20% of the vein bypass group and 18% of the best endovascular treatment group (adjusted HR, 1.23; 95% CI, 0.75-2.01).
°įThere are difficulties in conducting a trial involving the below-the-knee (BTK) arteries. Apart from the outcomes for the above-the-knee studies, which seem dependent upon patency and walking difficulties, the BTK data are mired in endpoints, heterogeneity of subjects, non-uniform nature of wound care, and type of patients enrolled.°Ī Garcia emphasized at the beginning of his lecture that these characteristics have been observed in endovascular studies involving BTK vessels, such as the IN.PACT DEEP, LEVENT, and SAVAL trials.
Emphasizing the features of the trials, Garcia mentioned that in the BEST-CLI study, only 18% of the enrollees were non-surgeons in the endovascular group, there was no interventional monitoring committee for those who did the intervention, and of the patients enrolled in the endovascular group, there was a 38% crossover that has never been defined. The primary endpoint was the composite of death and MALE. Of note, one component of MALE was a °ģmajor°Į reintervention, which did not include restenosis. Reintervention need and timing were determined by the site investigator, and there were no clinically-driven target lesion revascularization criteria or independent adjudication.
In BASIL 2, the MALE endpoints were similar in both treatment sides. Still, revision or primary procedural repeat as failure was assessed as an outcome, and any restenosis was considered a failure in the endovascular group. The primary endpoint was AFS or all-cause death. Of note, only mortality drove the difference between cohorts.
Garcia°Įs key message was that unfortunately, difficulties with patients, wounds, and endpoints have allowed no one trial to be successful and acceptable.
°įIn a given trial, patients need to be very specific and unless they are, the results will not answer the question for the cohort we see every day with CLTI. Unfortunately, that must be our first step. In this environment, the two trials are remarkable studies that, unfortunately, missed their mark for the question asked.°Ī Garcia concluded.
Hot Topics
EVAR, TEVAR and Peripheral Interventions
Saturday, April 27, 2:00 PM ~ 3:18 PM
Valve & Endovascular Theater, Level 2
Edited by

Pil Hyung Lee, MD
Seosan Jungang Hospital, Korea (Republic of)
