
Nicolas Van Mieghem
Erasmus University Medical Center, Netherlands
Patients with aortic stenosis (AS) have a high mortality risk across all levels of AS severity. Nicolas M. Van Mieghem, MD (Erasmus University Medical Center, Rotterdam, Netherlands) commented that any degree of AS is clinically relevant, and emphasized the role of cardiac remodeling, fibrosis, and dysfunction in the natural history of patients with AS, which yields high residual risk after aortic valve replacement (AVR).
Currently, AVR is only formally indicated for symptomatic, severe AS. However, optimal timing of intervention in patients with moderate AS remains controversial.
Mieghem mentioned that patients with concomitant moderate AS and left ventricular (LV) systolic dysfunction are also at high risk for clinical events such as death, AVR, or heart failure (HF) hospitalization, raising questions whether earlier AVR in these patients could improve clinical outcomes. Several studies have shown that patients with moderate AS and LV dysfunction are associated with a poor prognosis, as are patients with symptomatic AS.
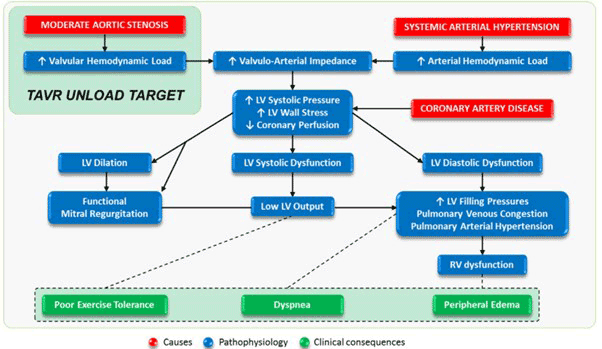
Transcatheter AVR (TAVR) can provide mechanical LV unloading in patients with HF and moderate AS. Based on the multinational ATLAS TAVI registry, patients with non-severe AS and reduced left ventricular ejection fraction had better survival with TAVR than with medical treatment. These results suggest the need for randomized controlled trials comparing TAVR versus medical treatment in patients with moderate AS and HF with reduced ejection fraction (HFrEF). Meanwhile, risk stratification approaches considering the presence of extra-aortic cardiac damage (i.e., LV, left atrial or mitral valve damage, pulmonary or tricuspid valve damage, right ventricular damage or subclinical HF) have also been studied, which may be helpful to identify asymptomatic AS patients who may benefit from elective AVR.
Several clinical trials are currently underway to answer the question of whether early AVR for moderate AS in the setting of HFrEF interrupts maladaptive cardiac remodeling and improves clinical outcomes. Among them, the results of TAVR UNLOAD trial (ClinicalTrials.gov: NCT02661451) are expected in 2024 (Figure 1). The PROGRESS trial (ClinicalTrials.gov: NCT04889872) and the Evolutʂ EXPAND TAVR II pivotal trial (ClinicalTrials.gov: NCT05149755) will also provide valuable insights on the moderate AS population.
Meet the Experts Over Breakfast
TAVR: Techniques and Issues
Friday, April 26, 7:30 AM ~ 8:25 AM
Presentation Room 1, Level 1
Edited by

Sangwoo Park, MD
Ulsan University Hospital, Korea (Republic of)
