
Hanbit Park
GangNeung Asan Hospital, Republic of Korea
The management of antiplatelet therapy in patients who need noncardiac surgery after percutaneous coronary intervention (PCI) with drug-eluting stents (DESs) requires consideration, including the risks of stent thrombosis with cessation and bleeding with continuation. The current guideline recommends continuation of aspirin perioperatively if the bleeding risk allows. However, for patients undergoing surgery with high bleeding risk (e.g. intracranial, spinal neurosurgery, or vitreoretinal ophthalmic surgery), discontinuation of aspirin is recommended at least 7 days preoperatively.
There are limited data on continuation of aspirin in patients with prior PCI with DES who are undergoing noncardiac surgery. The subgroup analysis of POISE-2 trial showed that in patients with prior PCI, continuation of aspirin reduced the risk of death or non-fatal myocardial infarction (MI) (hazard ratio [HR] 0.50; 95% confidence interval [CI] 0.26-0.95). The risk for major or life-threatening bleeding was neutral (HR 1.26; 95% CI 0.55-2.88). However, this study was underpowered and does not exclude a potential subgroup effect, as it was a subgroup analysis.
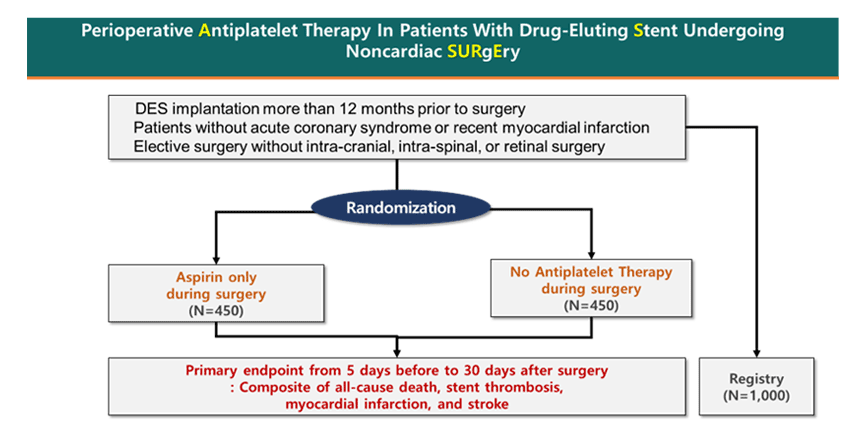
Perioperative Antiplatelet Therapy in Patients With Drug-eluting Stent Undergoing Noncardiac Surgery (ASSURE-DES) trial is an investigator-initiated, prospective, multicenter, randomized controlled trial comparing the safety and efficacy of aspirin cessation or continuation in perioperative period of noncardiac surgery in patients who have undergone PCI with DES for more than 12 months (Figure 1). Key exclusion criteria includes recent acute coronary syndrome (ACS) (within 1 month), severe left ventricular dysfunction (EF °¬ 30%), severe valvular heart disease, emergent operation, or high bleeding risk operation (e.g., intracranial, intraspinal, or retinal surgery). The primary endpoint was a composite of all-cause death, stent thrombosis, MI and stroke from 5 days before to 30 days after surgery.
From March 2017 through to March 2024, a total of 900 patients were enrolled. The primary results will become available this year, which is anticipated to provide valuable clinical evidence to determine optimal antiplatelet therapy in patients who underwent PCI with DES before noncardiac surgery.
Clinical Science
Ongoing Trials from Asan Medical Center
Saturday, April 27, 11:30 AM ~ 12:40 PM
Presentation Room 1, Level 1
Edited by

Hanbit Park , MD
GangNeung Asan Hospital, Korea (Republic of)
