
Do-Yoon Kang
Asan Medical Center, Republic of Korea
On April 27th, the primary results of comparing optical coherence tomography (OCT)-guided versus intravascular ultrasound (IVUS)-guided percutaneous coronary intervention (PCI) will be presented by Do-Yoon Kang, MD, PhD (Asan Medical Center, Ulsan University College of Medicine, Seoul, Korea), based on the insights from the Optical Coherence Tomography versus Intravascular Ultrasound-Guided Percutaneous Coronary Intervention (OCTIVUS) trial. The OCTIVUS trial was conducted by Duk-Woo Park MD, PhD et al., from 2018 to 2022, where 3897 and 2008 patients were screened and randomized, respectively. The results were reported at the European Society of Cardiology (ESC) congress in 2023 and published in Circulation.
To date, imaging-guided PCI has shown superior clinical outcomes compared to angiography-guided PCI. Therefore, it is recommended that IVUS or OCT be considered in selected patients to optimize stent implantation with a IIa level of evidence. However, controversies remain on the clinical efficacy and safety between OCT-guided and IVUS-guided PCI. Hence, the OCTIVUS trial was conducted to evaluate this issue.
The design of the pragmatic OCTIVUS Trial will be introduced in the session (Figure 1). It attempted to incorporate clinically relevant tools of usual intracoronary imaging in the routine PCI practice, a diverse study population with various clinical and anatomical characteristics, heterogeneous PCI management practice settings, use of a broad range of clinical endpoints, and lastly, clinically unmet issues in the daily clinical practice.
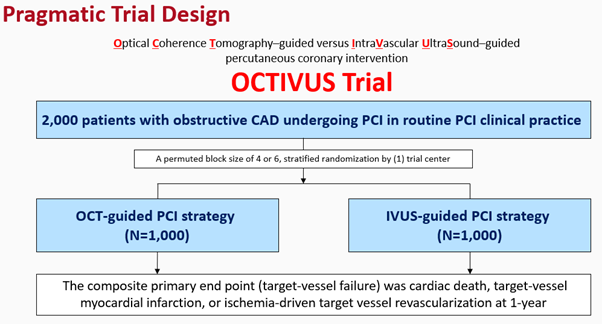
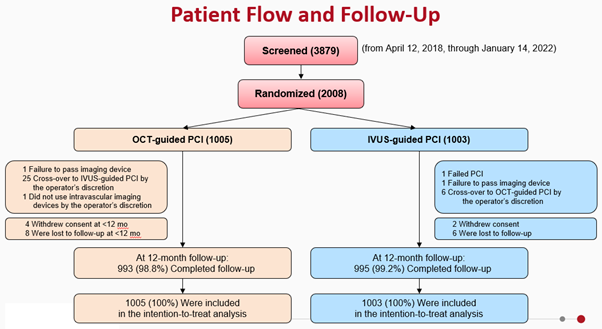
The primary endpoint of the trial was target vessel failure (TVF) at 1 year. Secondary endpoints included the individual components of the primary endpoint, target-lesion failure, stent thrombosis, repeat revascularization, contrast-induced nephropathy and procedural complications. Patient flow and follow-up scheme is provided in the figure below (Figure 2).
In the presentation, the results of the study will be shared, including key baseline characteristics, as well as anatomical and procedural characteristics, which successfully reflected a real-world clinical practice in a randomized-controlled trial (RCT) setting. Procedural outcomes and core lab-imaging analysis will also be provided to further the understanding of the results of the study. 53.4% and 60.1% of treated lesions met all stent-optimization criteria in the OCT-guided PCI group and IVUS-guided PCI group, respectively (p=0.001).
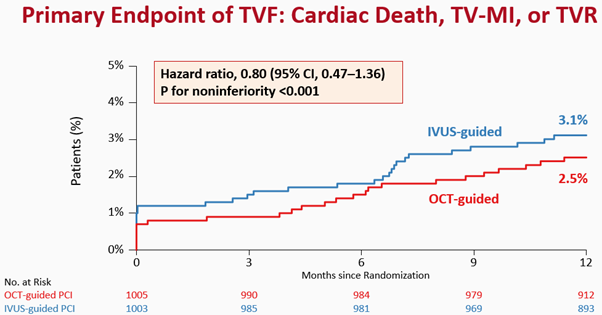
At 1 year after randomization, the primary endpoint, a composite of death from cardiac causes, target vessel-related myocardial infarction, or ischemia-driven target-vessel revascularization, had occurred in 25 of 1005 patients (2.5%) in the OCT-guided PCI group and in 31 of 1003 patients (3.1%) in the IVUS-guided PCI group (risk difference, −0.6 percentage points; upper boundary of the one-sided 97.5% confidence interval [CI], 0.97; P<0.001 for noninferiority) (Figure 3). The individual components of the primary endpoint and secondary endpoints will also be reported.
The presentation will culminate with the conclusion of the trial, that OCT-guided PCI was noninferior to IVUS-guided PCI with respect to a composite of death from cardiac causes, target-vessel myocardial infarction, or ischemia-driven target-vessel revascularization at 1 year. However, the selected study population and lower than expected event rates should be considered in interpreting the trial.
Clinical Science
Late-Breaking Clinical Trials 2024
Saturday, April 27, 8:30 AM ~ 10:03 AM
Presentation Room 1, Level 1
Edited by

Kyusup Lee, MD
The Catholic University of Korea, Daejeon St. Mary's Hospital, Korea (Republic of)
