Lots of interesting abstracts and cases were submitted for TCTAP 2024. Below are the accepted ones after a thorough review by our official reviewers. Don’t miss the opportunity to expand your knowledge and interact with authors as well as virtual participants by sharing your opinion in the comment section!
TCTAP A-050
Clinicodemographic and Left Ventricular Ejection Fraction Profile of Breast Cancer Patients Treated With Trastuzumab in a Tertiary Government Hospital
By Erwin Macaraeg Miranda, Edgar Christian Cuaresma, Kristianne Emmanuelle Bagaoiasan
Presenter
Erwin Macaraeg Miranda
Authors
Erwin Macaraeg Miranda1, Edgar Christian Cuaresma1, Kristianne Emmanuelle Bagaoiasan1
Affiliation
Dr. Paulino J. Garcia Memorial Research and Medical Center, Philippines1
View Study Report
TCTAP A-050
Pharmacotherapy (Heart Failure)
Clinicodemographic and Left Ventricular Ejection Fraction Profile of Breast Cancer Patients Treated With Trastuzumab in a Tertiary Government Hospital
Erwin Macaraeg Miranda1, Edgar Christian Cuaresma1, Kristianne Emmanuelle Bagaoiasan1
Dr. Paulino J. Garcia Memorial Research and Medical Center, Philippines1
Background
Breast cancer isone of the primary causes of mortality among women in both developed anddeveloping countries. The disease is a leading global cause of morbidity andmortality. According to Globocan 2020, there were 27,163 new cases of breastcancer and 9,926 fatalities in the Philippines. It is the most prevalent canceramong women in the country, and the incidence among Filipino women is thehighest in Southeast Asia. In developing nations, breast cancer has a negativeinfluence on quality of life due to decreased survival rates. Trastuzumab wasdeveloped in order to combat this lethal form of malignancy. It is a monoclonalantibody and restricting the human epidermal growth factor receptor 2 (HER2) isits mode of action. Trastuzumab was found to increase the overall survival ofpatients with this form of hormonal receptor breast cancer. However, its usehas been linked to cardiotoxicity in a subset of breast cancer patients.
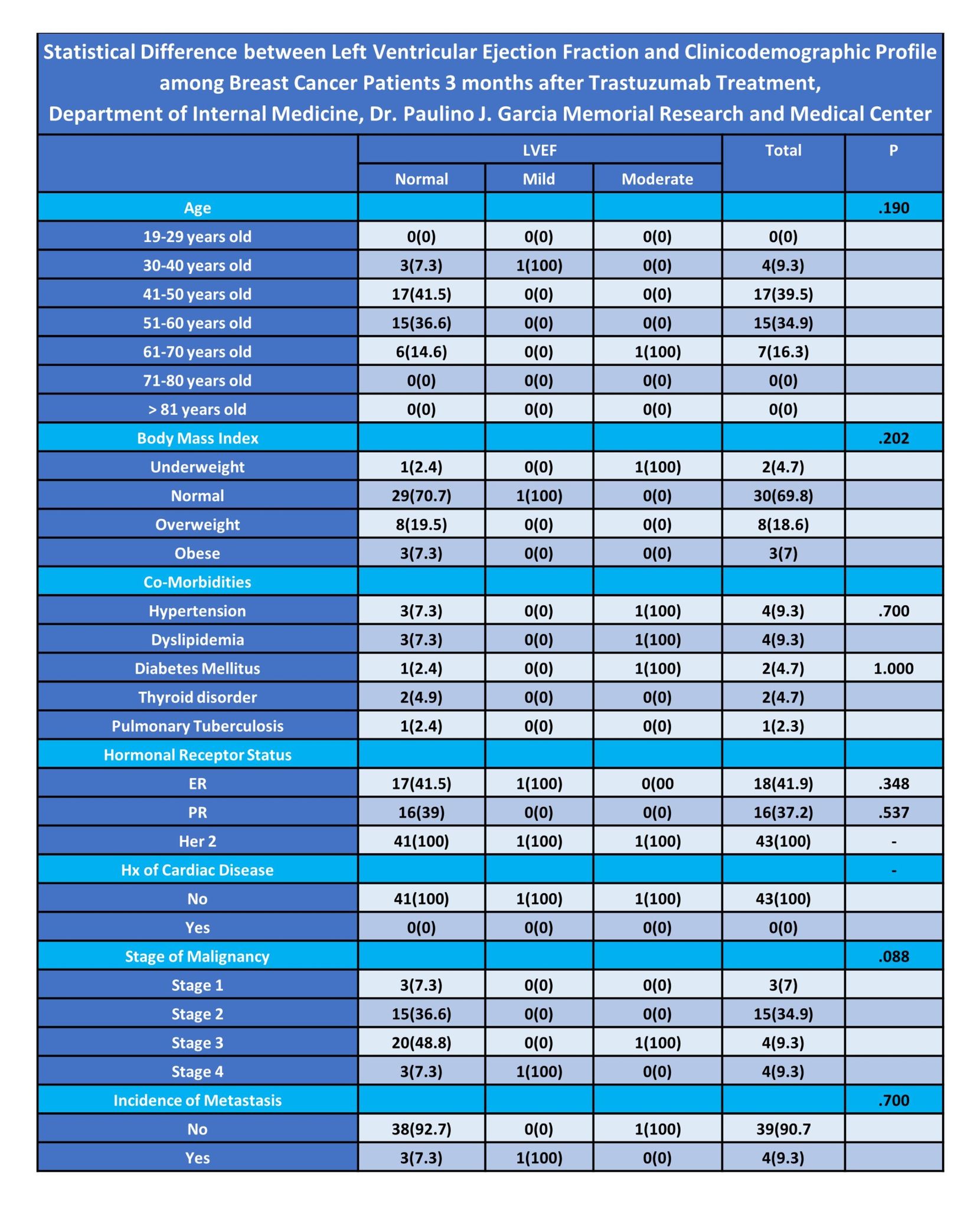
The general objective of thestudy is to determine the clinicodemographic and left ventricular ejectionfraction profile among breast cancer patients treated with Trastuzumab in aTertiary Government Hospital. The study includes several specific objectives.These objectives involve obtaining the clinicodemographic profile according toage, body mass index, presence of co-morbidities, hormonal receptor status,history of cardiac disease, stages of breast malignancy; and incidence ofmetastasis. The study also seeks to ascertain if there is significantdifference between the clinicodemographic characteristics and left ventricularejection profile among breast cancer patients after undergoing treatment withTrastuzumab for three months.
The general objective of thestudy is to determine the clinicodemographic and left ventricular ejectionfraction profile among breast cancer patients treated with Trastuzumab in aTertiary Government Hospital. The study includes several specific objectives.These objectives involve obtaining the clinicodemographic profile according toage, body mass index, presence of co-morbidities, hormonal receptor status,history of cardiac disease, stages of breast malignancy; and incidence ofmetastasis. The study also seeks to ascertain if there is significantdifference between the clinicodemographic characteristics and left ventricularejection profile among breast cancer patients after undergoing treatment withTrastuzumab for three months.
Methods
Retrospective cross-sectional research design was used in this study. Atotal of 43 breast cancer patients treated with Trastuzumab were included inthe study. Data obtained were patients’age, body mass index, presence of co-morbidities, hormonal receptor status,history of cardiac disease, stage of breast malignancy and incidence ofmetastasis. After collecting the necessary data, the baseline left ventricularejection fraction(LVEF) was then recorded. The researcher also recorded theLVEF 3 months after the patients started receiving Trastuzumab. The baselineLVEF was compared to the 2D echo taken at the 3-month mark of treatment.Patients 2d echo results were classified according to the categories of LVEF:normal, mild dysfunction, moderate dysfunction, severe dysfunction. Frequency,percentage and distribution were used to obtain the clinic-demographic profileof breast cancer patients. Chi Square Test was be used to compare the left ventricularejection fraction profile of breast cancer patients after three months ofundergoing treatment for Trastuzumab.
Results
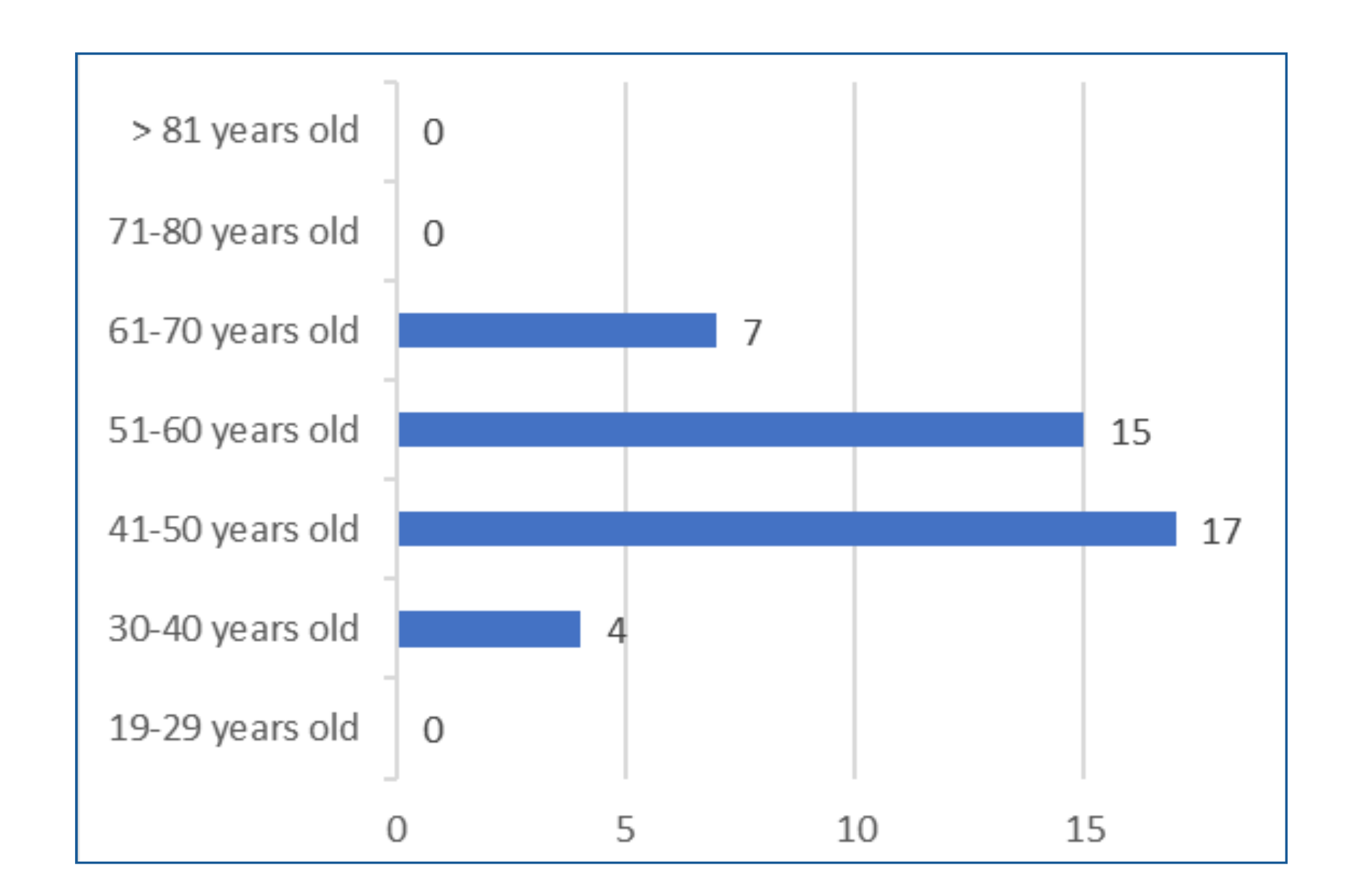
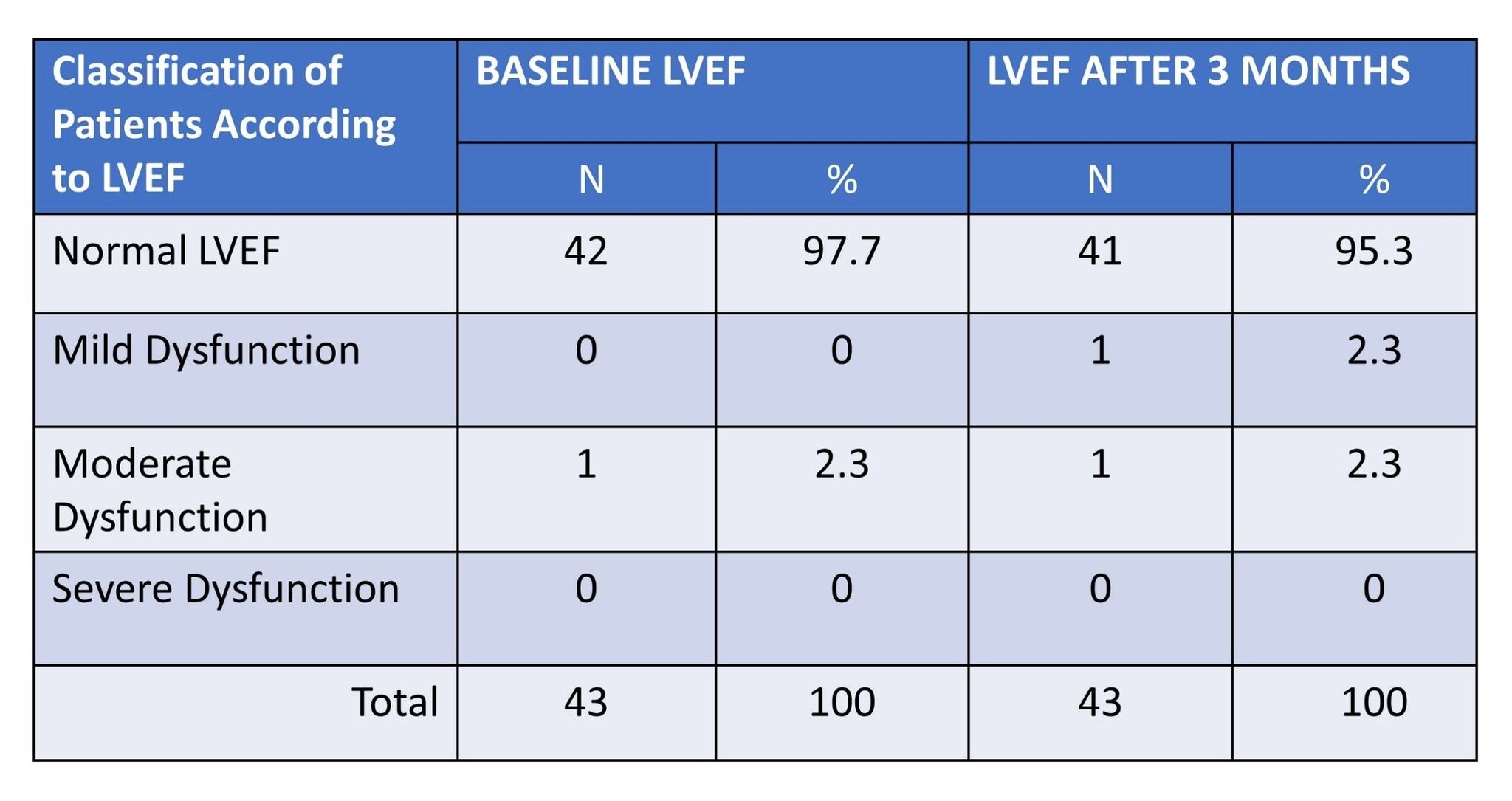
The study revealed that almostall breast cancer patients included in the study before the TrastuzumabTreatment had normal left ventricular ejection fraction (42;97.7%). On theother hand, only 1 (2.3) patient had moderate LVEF dysfunction. Further, in theresearch study, it also revealed that the majority of cases had normal LVEF(41; 95.3%). Only 1 (2.3%) case of LVEF mild dysfunction and moderatedysfunction (1; 2.3%) were recorded in the study. There were no cases of severedysfunction LVEF in patients placed under Trastuzumab for 3 months. The highestnumber of cases (39.5%) fell within the 41 to 50 years age group, followed by34.9% in the 51 to 60 years age group. Among the patients, 69.8%were categorizedas normal weight, with 18.6% being overweight, and only 7%obese. Hypertension(9.3%) and dyslipidemia(9.3%) were the most common co-morbidities. All patients(100%) were tested for positive HER2 hormonal receptor. None of the patientshad a history of cardiac disease. Most patients were in stage 3malignancy(48.8%), followed by stage 2 (34.9%). Before Trastuzumab treatment, nearly allpatients (97.7%) had normal LVEF. After 3 months, the majority maintainednormal LVEF (95.3%), with 2.3% having mild LVEF dysfunction and 2.3%havingmoderate dysfunction. The clinicodemographic variables did not significantlyaffect LVEF outcomes.



Conclusion
The study revealed that 3 months of Trastuzumab treatment did not yieldany significant cardiotoxicity in patients with breast cancer. Furthermore,baseline LVEF of patients did not show any statistical variation in terms ofage, body mass index, co-morbidities, hormonal receptor status, history ofcardiac disease, stage of malignancy and incidence of metastasis. The resultsclearly indicate that while monitoring may be needed as regards its use. Thebenefit of Trastuzumab as a good adjuvant for breast cancer therapy should notpreclude treatment. It is recommended that clinicians should closely monitorbreast cancer patients treated with Trastuzumab even it was found to be notcardiotoxic when used for the first three months. This ensures that any adverseeffects related to this drug is addressed immediately. Further imagingmodalities such as Global Longitudinal Strain and Multigated Acquisition (MUGA)scan can also be utilized in identifying early and subclinical alterations inleft ventricular ejection fraction among breast cancer patients treated withTrastuzumab.

