Lots of interesting abstracts and cases were submitted for TCTAP 2024. Below are the accepted ones after a thorough review by our official reviewers. Don’t miss the opportunity to expand your knowledge and interact with authors as well as virtual participants by sharing your opinion in the comment section!
TCTAP A-031
Effectiveness and Safety of Polymer-Free Biolimus-Eluting Stents (PF-BES) in Thailand
By Wongsakorn Luangphiphat, Thamarath Chantadansuwan, Akaphol Kaladee
Presenter
Wongsakorn Luangphiphat
Authors
Wongsakorn Luangphiphat1, Thamarath Chantadansuwan2, Akaphol Kaladee3
Affiliation
Chulabhorn Hospital, Thailand1, Central Chest Institute of Thailand, Thailand2, Sukhothai Thammathirat Open University, Thailand3
View Study Report
TCTAP A-031
DES/BRS/DCB
Effectiveness and Safety of Polymer-Free Biolimus-Eluting Stents (PF-BES) in Thailand
Wongsakorn Luangphiphat1, Thamarath Chantadansuwan2, Akaphol Kaladee3
Chulabhorn Hospital, Thailand1, Central Chest Institute of Thailand, Thailand2, Sukhothai Thammathirat Open University, Thailand3
Background
An estimated 20% of patients undergoing percutaneous coronary intervention (PCI) are at high bleeding risk (HBR) with associated mortality hazards. Most of them are excluded from many stent trials. In a first-in-human evaluation, the Biolimus-eluting stent was non-inferior to a Paclitaxel-eluting stent for in-stent late lumen loss at 12 months. Since then, numerous publications have been reporting using the Polymer-free Biolimus-eluting stents (PF-BES), mainly on HBR patients. However, there is a lack of data on the clinical outcomes of effectiveness and safety of PF-BESs in Thailand.




Methods
An observational, retrospective utilizing chart review, single-arm study was conducted. All consecutive patients who underwent PCI and received PF-BES at CCIT from 1st August 2015 to 31st July 2020 were included in this analysis.
Results
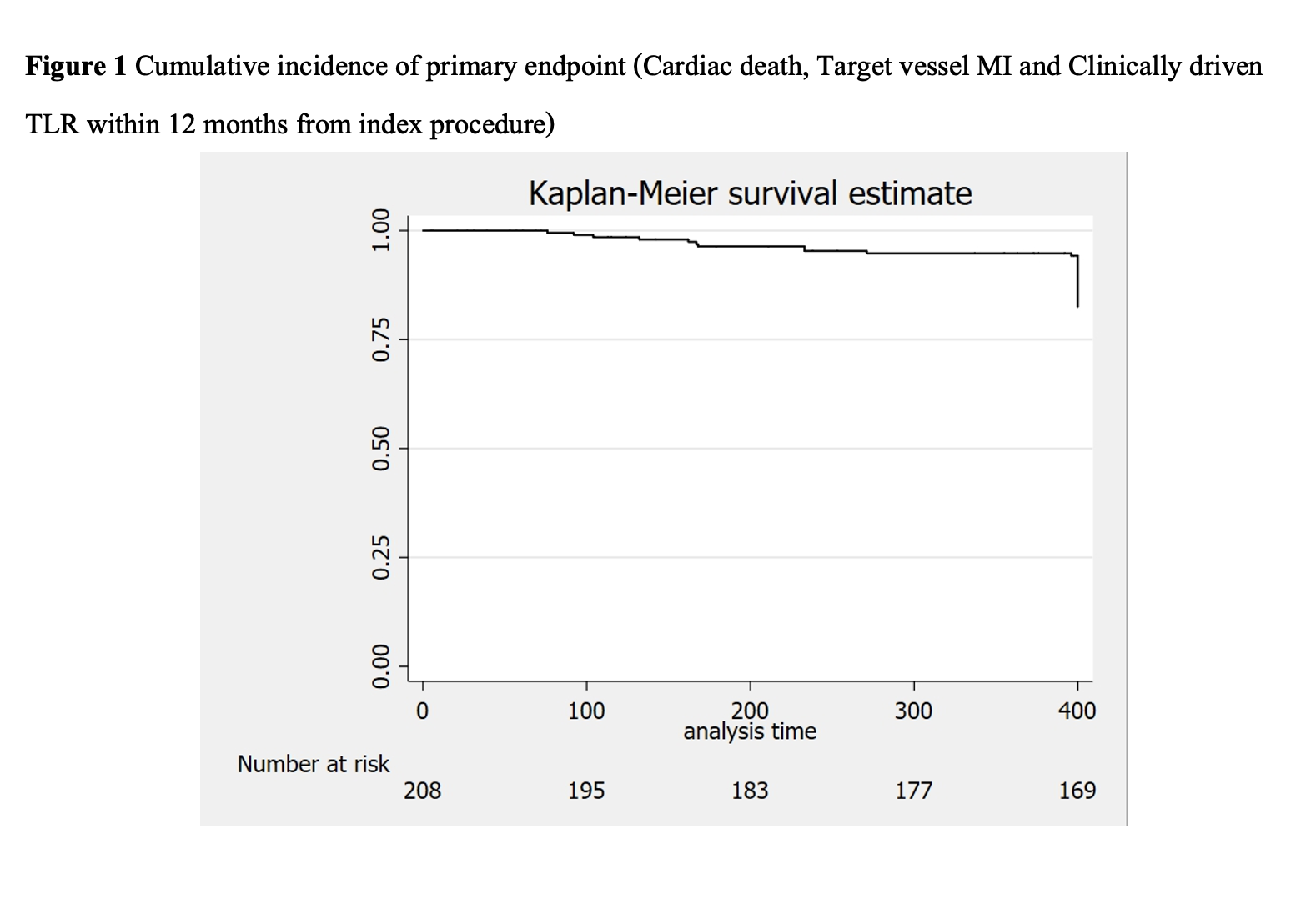
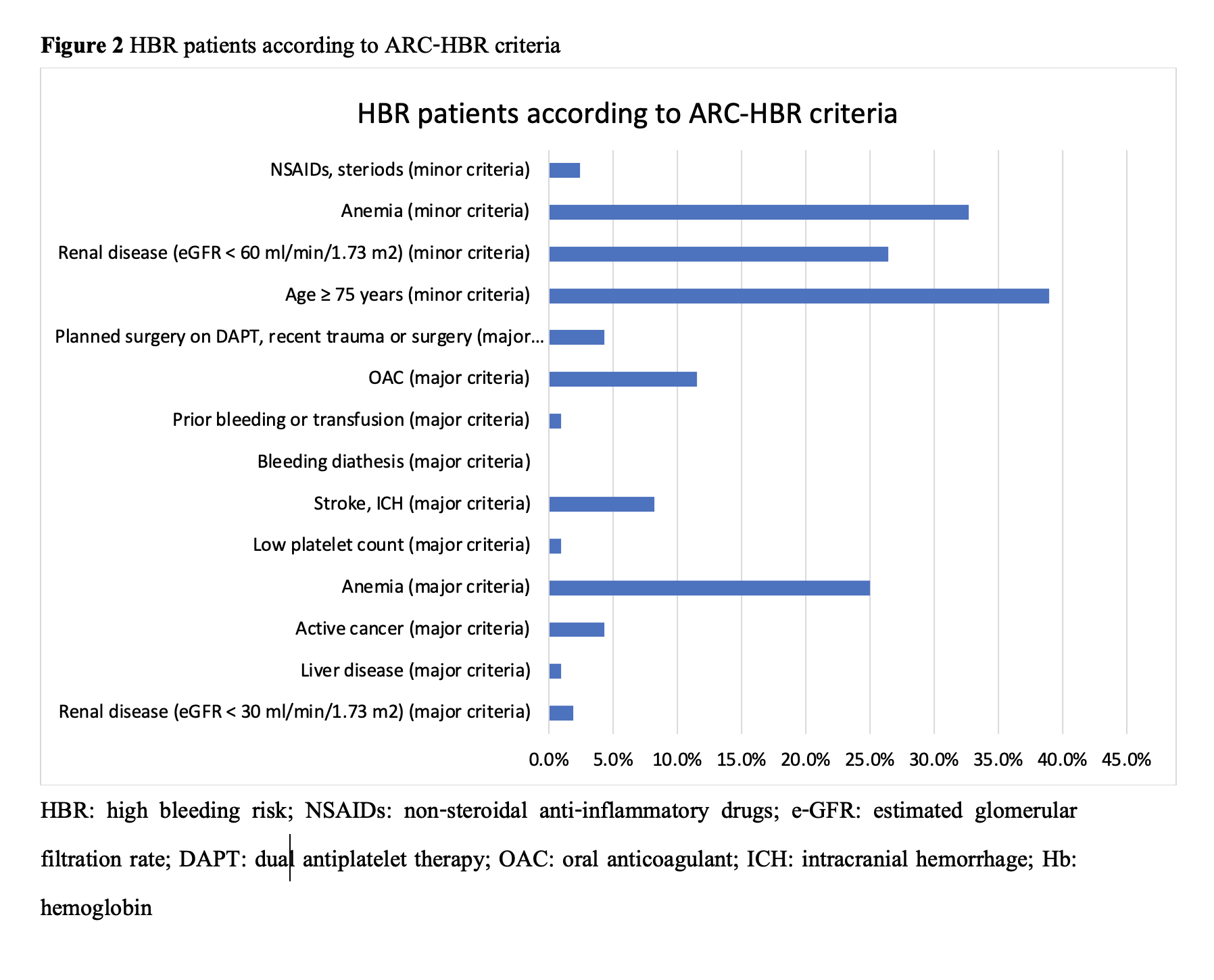
Baseline characteristics of 208 patients who received PF-BES were almost male (66.3%), with a mean age of 70.4±12.8 years. The patients’ risk factors were dyslipidemia (93.3%), hypertension (84.1%), and diabetes mellitus (41.8%). The patients who underwent PCI for indication of chronic coronary syndrome, STEMI, NSTEMI, and unstable angina were 59.6%, 24.7%, 14.6%, and 0.7%, respectively. The mean CRUSADE score was 36.1±14.9, and 30.3% of patients had score >40 points, categorized as having a high risk of bleeding. Half of the patients (54.3%) had high bleeding risk according to ARC-HBR criteria. Eighteen patients were on warfarin with a median time in therapeutic range (TTR) of 37.5±28.0%. The left anterior descending artery was the most frequently treated target vessel (40.1%). The mean SYNTAX score was 28.1±14.7. PF-BESs were used 21.4% in ostial lesions, 21.0% in bifurcations lesions, and 38.7% in calcified lesions. According to the ACC/AHA lesion classification system, most lesions were classified as type B (90.6%). Dual antiplatelet therapy (DAPT) was prescribed for 88.5% of patients at discharge and 69.2% at one year follow up, while triple therapy was used 11.5% at discharge and 7.2% at one year follow up. In our study, the mean DAPT duration was 365.5±87.3 days.
Conclusion
In terms of device effectiveness, when we used Polymer-free Biolimus-eluting stent (PF-BESs) in a real-world situation, our study showed less unfavorable outcomes such as cardiac death, MI, TV-MI, and clinically driven TLR compared to the LEADERS FREE results. PF-BES is safe and effective in real-world patients irrespective of HBR status.

