Lots of interesting abstracts and cases were submitted for TCTAP & AP VALVES 2020 Virtual. Below are accepted ones after thoroughly reviewed by our official reviewers. Don¡¯t miss the opportunity to explore your knowledge and interact with authors as well as virtual participants by sharing your opinion!
* The E-Science Station is well-optimized for PC.
We highly recommend you use a desktop computer or laptop to browse E-posters.
CASE20200410_004
| STRUCTURAL HEART DISEASE - Valvular Intervention: Aortic | |
| Transcatheter aortic valve implantation with a novel balloon expandable valve for rheumatic aortic stenosis in a patient with a prosthetic mitral valve | |
| John Jose Erungaren1, Pradeep Kumar Rajnala | |
| Christian Medical College Hospital, India1, | |
|
[Clinical Information]
- Patient initials or identifier number:
AK
-Relevant clinical history and physical exam:
Dyspnoea of 1year duration; presently NYHA classII. Diagnosed to have severe aortic stenosis. Previous mechanical mitral valvereplacement 15 years back. Known CKD. Recurrent subdural hematoma- frontotemporoparietal chronic subdural hematoma and underwent rightfrontal burr hole and evacuation elsewhere on 20/09/2019. Right frontal burrhole re-exploration and right parietal burr hole and evacuation of recurrentchronic subdural hematoma on 17/10/2019. Heart team decision for TAVR
-Relevant test results prior to catheterization:
ECG-atrialfibrillation.
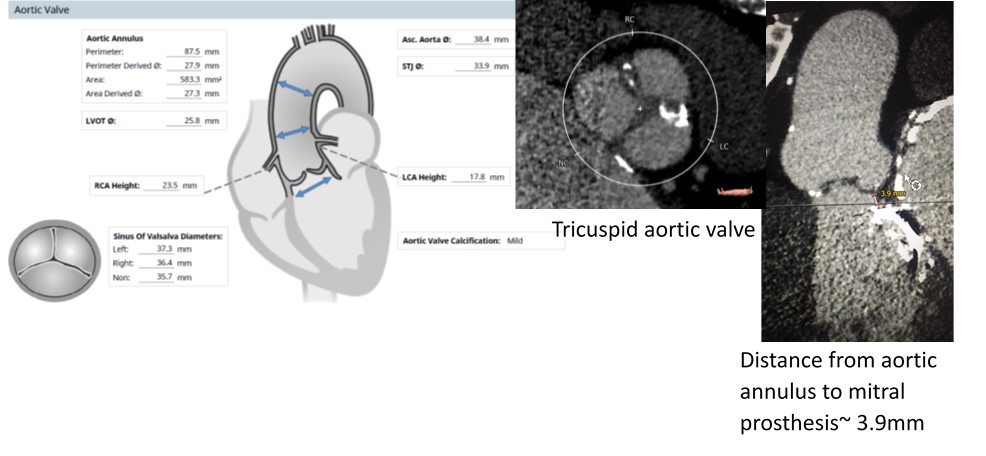 - Relevant catheterization findings:
|
|
|
[Interventional Management]
- Procedural step:
Transfemoral route transcatheter aortic valveimplantation was done under conscious sedation. 2 proglides. During valve crossing, care was taken to ensure the wire did not pass through the mechanical mitral prosthesis (by observing in orthogonal views). View for pre dilatation was the angulation that showed maximum separation of the balloon. Pre-dilatation was done to assess balloon shifts and impression on the mechanicalmitral valve. A 27.5mm Myval (Meril lifesciences)- a novel balloon expandable valve, with additional 2ml volume added inthe balloon was deployed by very slow balloon inflation. Fluoroscopy time-27:58 min. Contrast volume- 135ml Visipaque.
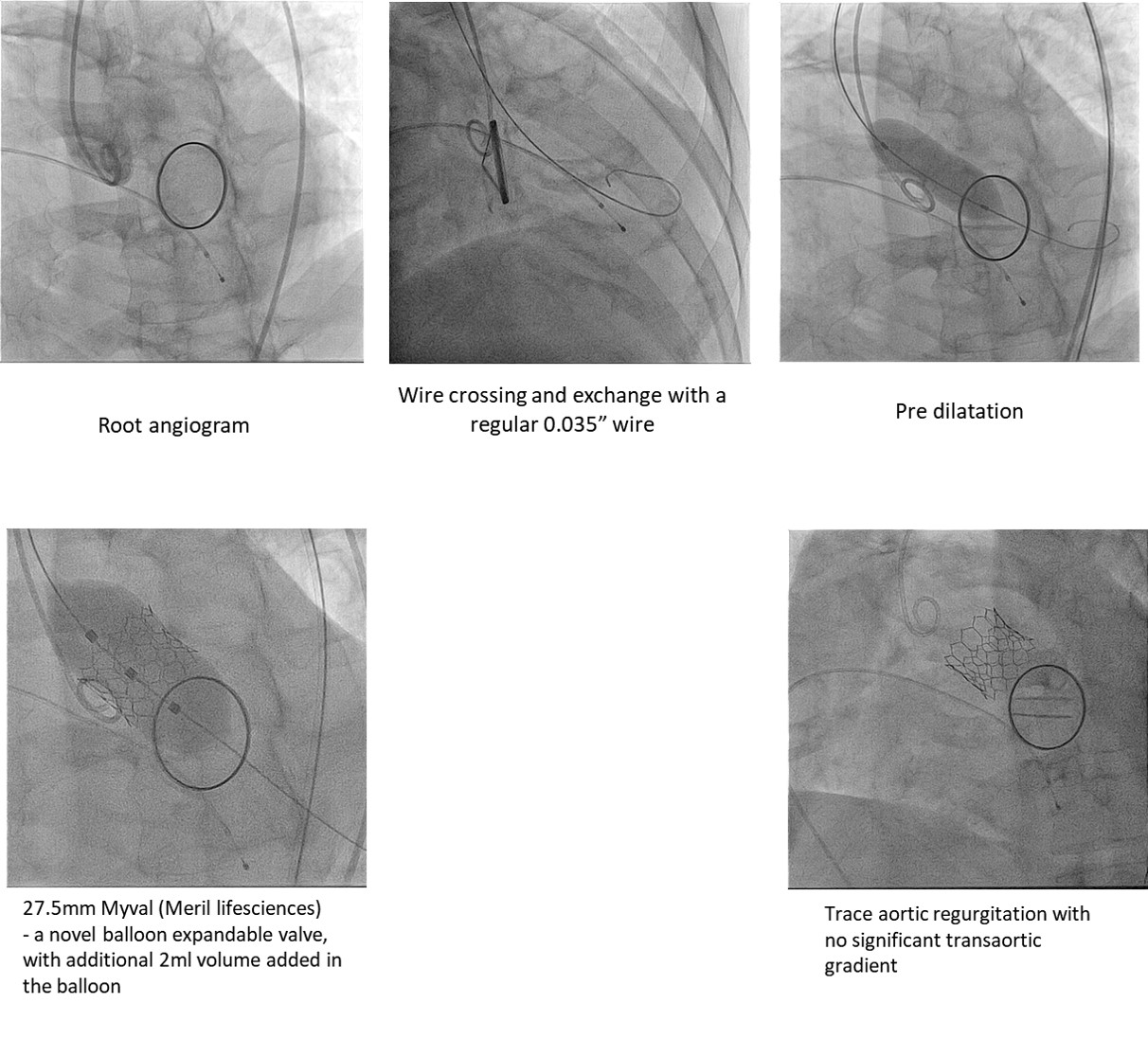 - Case Summary:
Post procedure-Forcedsaline diuresis and fluid replacement, Serum creatinine 1-week post discharge-3.20 mg/dl (baseline-3.6mg/dl). Pre discharge ECHO- mean gradient across aortic valve: 13.0 mmHg;valve area- 2.0 cm2. NYHAclass 1 at discharge.
This case highlights feasibility of transcatheter aorticvalve implantation with a novel balloon expandable valve in a patient withaortic stenosis and prior mechanical mitral prosthesis with a short aorticannulus-mitral prosthesis distance. |
|