Lots of interesting abstracts and cases were submitted for TCTAP & AP VALVES 2020 Virtual. Below are accepted ones after thoroughly reviewed by our official reviewers. Don¡¯t miss the opportunity to explore your knowledge and interact with authors as well as virtual participants by sharing your opinion!
* The E-Science Station is well-optimized for PC.
We highly recommend you use a desktop computer or laptop to browse E-posters.
ABS20191031_0002
| Basic Science, Animal Models and Preclinical Studies | |
| Discovery and validation of plasminogen activator inhibitor-1 platelet-derived extracellular vesicles to predict major adverse cardiac events | |
| Richard Gregory Jung1, Trevor Simard1, Shan Dhaliwal1, Pietro Di Santo1, Simon Parlow1, Omar Abdel-Razek1, Mina Rizk1, Joanne Joseph2, Caleb Sypkes1, Robert Moreland1, Anne-Claire Duchez1, Dylan Burger3, Benjamin Hibbert1 | |
| University of Ottawa Heart Institute, Canada1, The Ottawa Hospital, Canada2, Ottawa Hospital Research Institute, Canada3 | |
|
Background:
Coronary revascularization and optimal risk factor management remains central for management of obstructive coronary artery disease. However, identification of patients at risk for adverse events following coronary angiography remains limited despite the development of clinical risk scores. Furthermore, extracellular vesicles (EVs) are pro-thrombotic particles which are elevated in myocardial infarction and may serve as a potential biomarker of major adverse cardiac events (MACE). This study sought to evaluate the utility of plasminogen activatorinhibitor-1 positive platelet-derived extracellular vesicles (PAI-1+PEV) as a biomarker for major adverse cardiac events (MACE) followingangiography.
|
|
|
Methods:
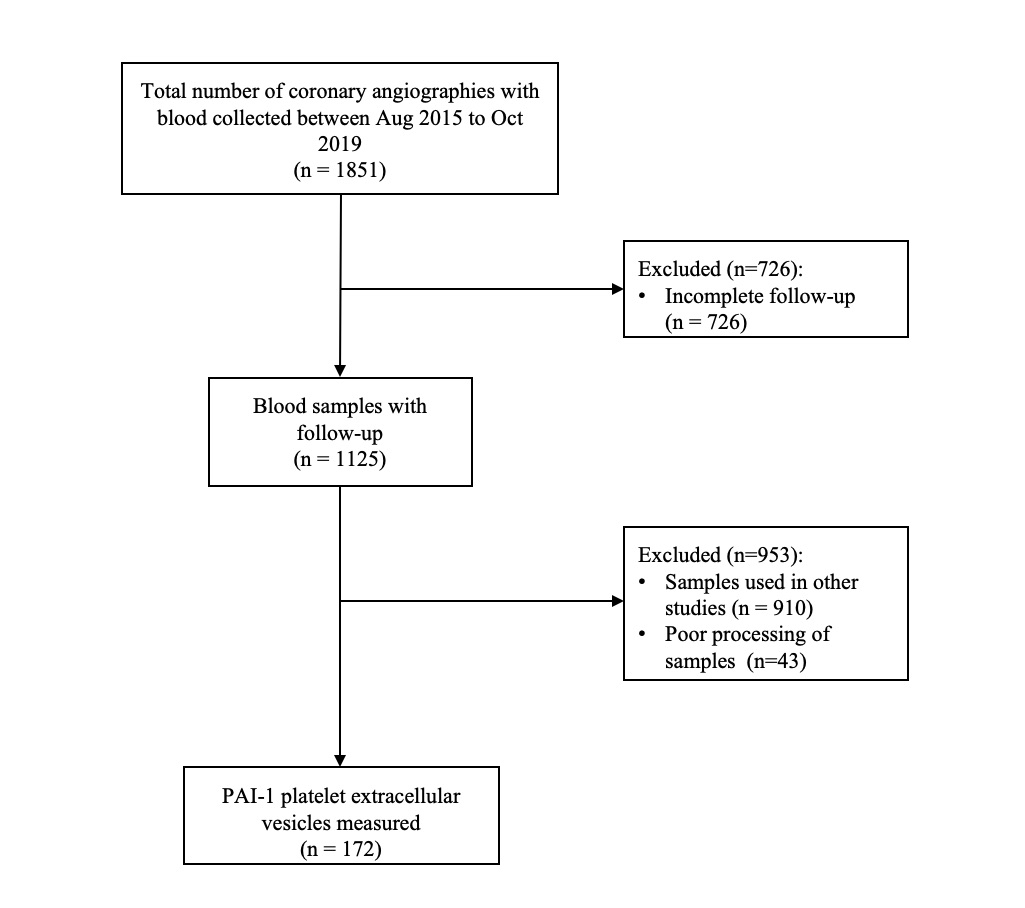
The University of Ottawa Heart Institute is a regional tertiary cardiac care center in the capital region of Canada. The CAPITAL Revascularization Registry is used to capture baseline procedural and patient data. Standardized clinical follow-up is completed at one-year for clinical outcomes. Plasma was collected from patients at the time of catheterization and frozen at -80 degrees Celsius until EV analysis. Platelet PAI-1 EV (Annexin V+/CD41+/PAI-1+EVs on flow cytometry) was isolated from plasma by centrifugation at 20,000 x g, stained, and measured by flow cytometry. A ROC curve was generated in order to evaluate the performance of platelet PAI-1 EVs as a biomarker to identify patients at-risk for MACE.
 |
|
|
Results:
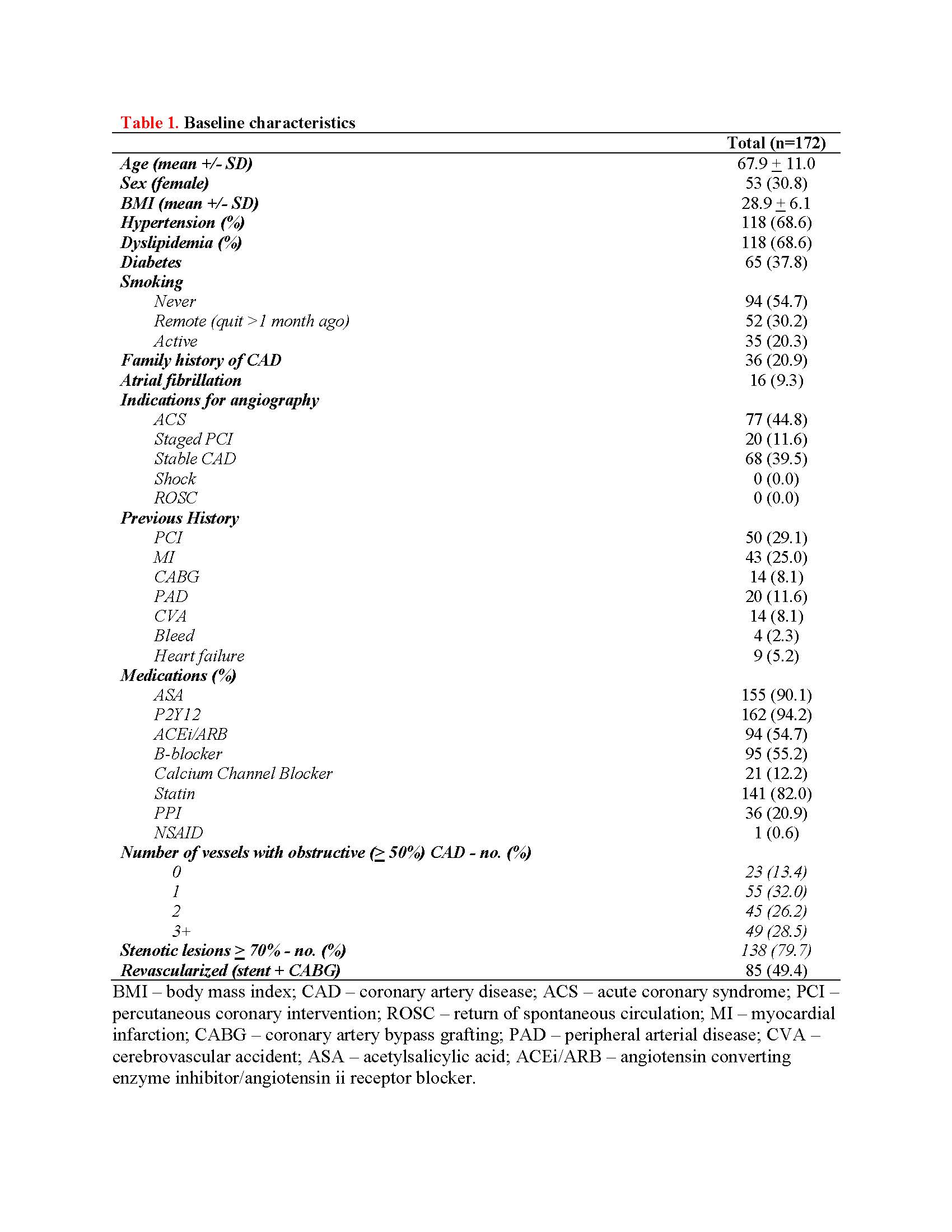
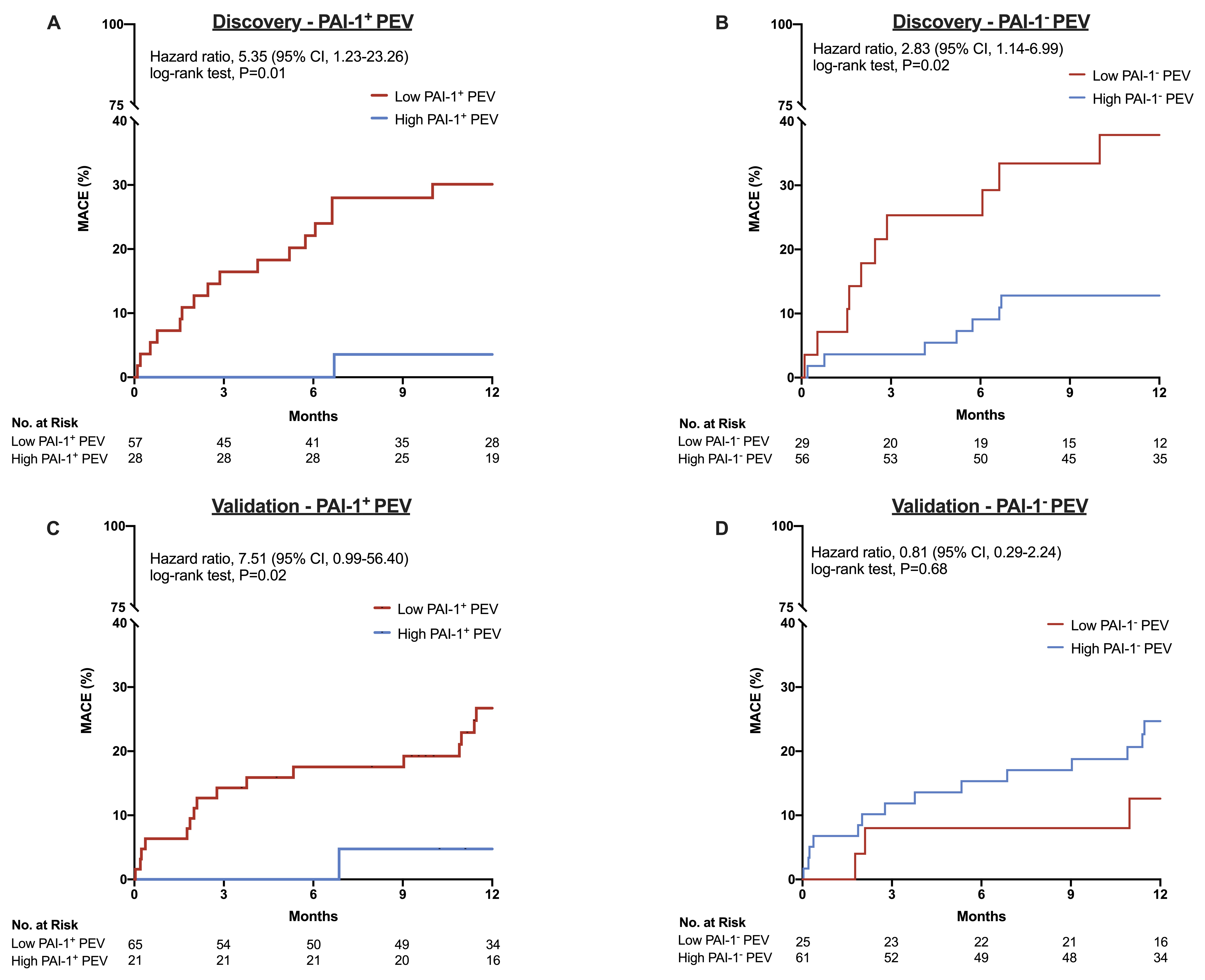
From August 2016 to August 2019, a total of 1851 patients had completed one-year follow-up with frozen plasma. Of these, 172 (9.3%) patients had their extracellular vesicles measured by flow cytometry. Duringa median follow-up period of 377 days (IQR, 269.5 to 442.5 days), 38 patients(20.9%) experienced MACE. The mean age of our cohort was 67.9 +/- 11.0 years and 69.2% were male. 85 (49.4%) patients were referred for CABG or underwent PCI at the time of coronary angiography. 65 (37.8%) patients had type 2 diabetes, 118 (68.6%) had hypertension and dyslipidemia, 35 (20.3%) were active smokers, and 77 (44.8%) were diagnosed with acute coronary syndrome (ACS) at time of angiography. Lowlog-transformed PAI-1+ PEV levels were associated with MACE (4.17 +/- 0.40 vs. 4.33 +/- 0.59 logPAI-1+PEV, p=0.02). After adjustment for known clinical risk factors, low PAI-1+ PEV levels were independently associated with MACE with a hazard ratio of 7.79 (95% CI, 1.87 to 32.4, p=0.0005). Finally, low plasma PAI-1+ PEV levels were predictive of MACE in both the discovery and validation cohort.
  |
|
|
Conclusion:
Our results demonstrate the existence of a PAI-1+PEV and its potential utility as a biomarker to predict MACE. Low plasma PAI-1+PEV levels were predictive of MACE in both the discovery and validation cohort.
|
|